Fuel cells are becoming an increasingly important technology for electrical energy, especially for long-haul trucks and as backup power sources for homes. They convert hydrogen, which is increasingly produced using renewable energy sources with low greenhouse gas emissions, into electrical energy without emitting carbon dioxide. However, that does not mean they do not have an impact on the environment.
Using fluorine in the plastic membranes that allow ions to move from one side of a fuel cell to the other comes with a cost, both as an expensive materials component and as a toxic pollutant. Nonetheless, fluorine-based polymer membranes, made from a material known as PFSA, are currently unmatched when it comes to compatibility with the cell’s electrodes and high conductivity for protons or hydroxide ions traveling between them, essential traits of a long-lasting, safe and efficient fuel cell.
With bans on the chemical being proposed in the U.S. and around the world, the challenge now is creating fluorine-free polymer membranes that do the same job.

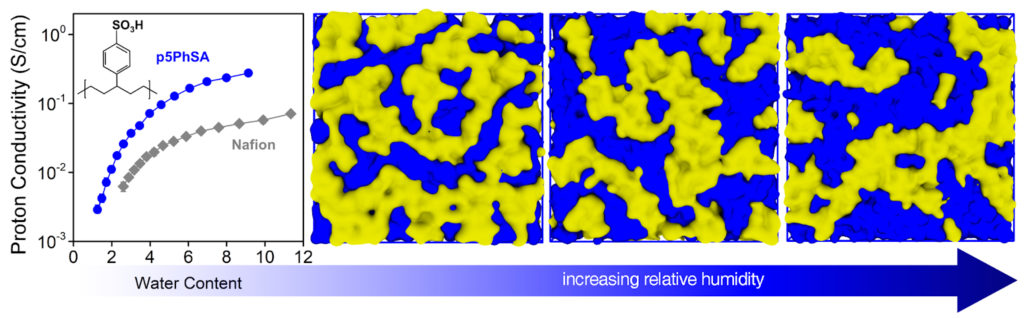
Karen Winey, Harold Pender Professor of Chemical and Biomolecular Engineering and Materials Science and Engineering, has already started to address this challenge by using fluorine-free polymers with precisely placed sulfonate groups. When these proton exchange membranes (PEMs) come into contact with water, the sulfonate groups assemble to form intricate water channels for protons to flow through.
This change in the chemistry of the electrolyte removes the need for fluorine altogether, but there are areas that need further investigation to develop and ensure this solution is viable.
Winey has now been awarded a Department of Energy grant that will provide $2.2 million over three years to fund the design, synthesis and study of hydrocarbon-based PEMs to mimic the relevant features of PFSA membranes. Obtaining a better understanding of the fundamentals of the membrane structure and dynamics will help the team determine the next steps to developing even cleaner and more efficient fuel cell technologies.
For this research, Winey will collaborate with Amalie L. Frischknecht of Sandia National Laboratories, Michael A. Hickner of Pennsylvania State University, and Justin G. Kennemur of Florida State University.
“Our team has the combined skills to establish the role of the local structure and dynamics at the hydrophobic/hydrophilic interface on proton and hydroxide transport in these new and important materials,” says Winey. “Our research requires synthetic control and versatility to modify the molecular structure, which is available in Kennemur’s group using ring-opening metathesis polymerization, or ROMP, and subsequent functionalization. As recently determined by Frischknecht’s all-atom molecular dynamics simulations and corroborated by X-ray scattering studies conducted by my group, the linear saturated carbon backbones of these polymers have sufficient flexibility to form well-developed percolated water domains.”
Frischknecht and Winey have previously combined simulations, electrochemical impedance spectroscopy and quasielastic neutron scattering studies to extract new insights about ion and chain dynamics. With the addition of Hickner’s expertise in infrared and nuclear magnetic resonance spectroscopies, the team will be able to reveal an even finer-grained picture of these polymers’ internal structure, elucidating the fundamental role of water in determining their ion transport properties.
“Detailed understanding of how protons and hydroxide ions move in hydrated polymers is only possible through this kind of highly coordinated effort,” says Winey.
This collaborative DOE grant is the second that Winey has led in as many years. In August 2022, Winey launched a new $3.25 million grant with collaborators at Penn and the University of Massachusetts, Amherst focusing on developing polymer-to-polymer conversion to reduce polymer waste. Together these efforts reflect Penn Engineering’s commitment to building a more sustainable future.
