Penn Engineers Develop Bone-like Metal Foam that can be ‘Healed’ at Room Temperature
For 6,000 years, humans have been making things from metal because it’s strong and tough; a lot of energy is required to damage it. The flip side of this property is that a lot of energy is required to repair that damage. Typically, the repair process involves melting the metal with welding torches that can reach 6,300 °F.
Now, for the first time, Penn Engineers have developed a way to repair metal at room temperature. They call their technique “healing” because of its similarity to the way bones heal, recruiting raw material and energy from an external source.

The study was conducted by James Pikul, assistant professor in the Department of Mechanical Engineering and Applied Mechanics and Zakaria Hsain, a graduate student in his lab.
It was published in the journal Advanced Functional Materials.
Beyond the energy costs associated with the current process of repairing metal by melting it to a more pliable form, there are some metal components where such a repair strategy is not even an option. For example, melting removes the intricate internal structure of metallic foams, which are metals made with internal pockets of air. This arrangement of struts and gaps reduces the material’s weight while maintaining its overall strength.
While exploring ways to repair such porous metals, Pikul and Hsain looked at existing “self-healing” materials, which are generally made from relatively soft polymers and plastics.
“The way people do self-healing today is they impregnate these polymers with different chemicals that, when that polymer is ruptured, are released and mix like an epoxy, gluing the material back together,” Pikul says. “That approach works for polymers because polymers can flow and are relatively easy to reshape at room temperature, but that means they have limited strength as a result.”
To heal metal foams, which generally have better structural properties than polymers, Pikul and Hsain started by finding a way for them to “sense” where they had been damaged. Rather than encapsulating additional chemicals used in repair, the researchers realized that they could use the breaking of a polymer layer as a kind of chemical signal.
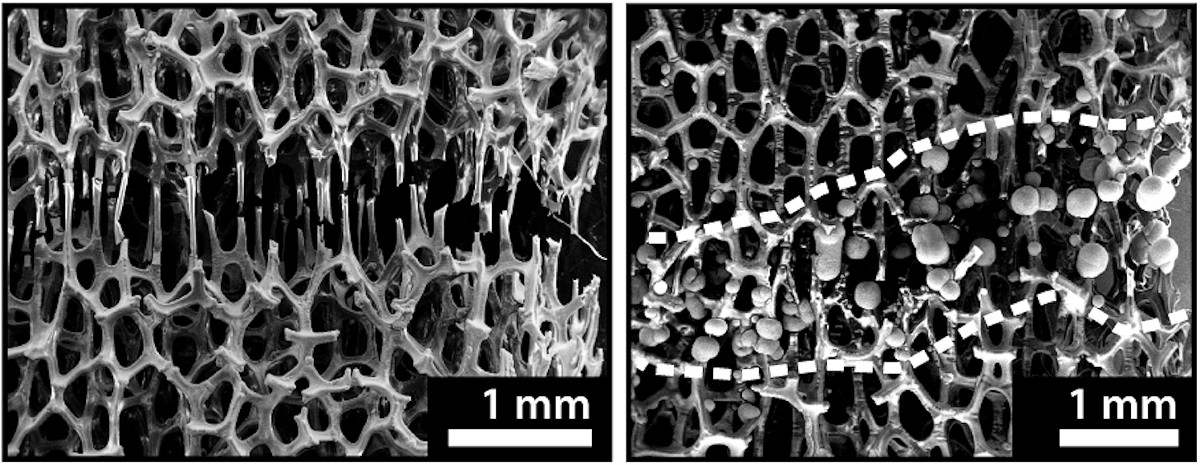
Pikul and Hsain used chemical vapor deposition to evenly coat each strut of the nickel foam with a layer of Parylene D, a chemically inert and stretchy polymer. Because this material’s damage tolerance is slightly lower than that of nickel, it breaks first as the sample is damaged, revealing the metal underneath. The researchers could then use electroplating to build new nickel struts only on the exposed nickel where they were needed.
Electroplating is a relatively low-energy, room-temperature technique, most commonly used to add a layer of chrome to car parts or gold to jewelry. In the former example, a steel tire rim is placed in a liquid electrolyte bath that contains chromium ions. When a voltage is applied, ions near the steel react and form a uniform chromium metal coating on the steel.
“Unlike polymers, metals don’t flow at room temperature,” Pikul says. “The nice thing with electrochemistry is that metal ions can easily move through the liquid electrolyte. We then use electrochemistry to convert the ions to solid metal. The polymer acts like a lithography mask and only allows the ions to turn into metal where the metal foam was broken.”

Pikul and Hsain healed three types of damage in their experiments on centimeter-scale samples of their polymer-coated nickel foam: samples with cracks, samples that had been pulled apart until they were connected by just a few struts, and samples that had been cleaved in two.
Healing the damage took about four hours, and because electroplating acts on all of the exposed nickel at once, the time it takes to heal damage is independent of the sample’s size.
While this room-temperature approach is not truly “self-healing” because it requires an external power source and raw materials, Pikul sees it being in line with how self-healing occurs in the body.

“I think most people would say bone is a self-healing material,” Pikul says, “and I think, in practice, our material is very similar to bone. Bone is not fully self-contained either; it needs an energy source and nutrients to heal, both of which come from eating food. In our system, these function similarly to the voltage and electroplating bath.”
Also like bone, the repaired areas are actually stronger than they were before they were damaged because extra nickel is grown at the healing site. The new nickel, however, reduces the healing efficiency when repeatedly using this technique. Because the healed areas no longer have a polymer coating, nickel would continue to amass there should another piece of the sample need to be healed.
Pikul hopes that further research on this technique will increase the similarities to biological healing.
“The electrolyte fluid that allows the healing can be integrated into the metal foams so that it resembles blood in our body,” Pikul says. “Once the foam is fractured, the electrolyte will surround the fractured area and heal the metal after the application of an external voltage, which can be from a battery.”
The foam could be healed without having to remove and submerge the damaged part — particularly useful if the part in question is a car door, robotic arm, or space-station component.
The research was supported by the National Science Foundation through a National Nanotechnology Coordinated Infrastructure program grant NNCI‐1542153.
