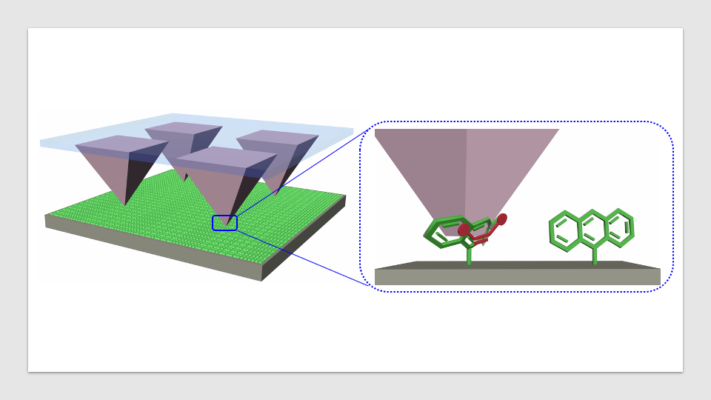
The production of chemicals accounts for 40% of all energy currently used in manufacturing, and the process also results in toxic solvent waste that pollutes the environment and poses health risks to humans and animals. A newly published study in the journal Science details a novel mechanochemistry method that has the ability to manufacture chemicals without those deleterious effects.
Researchers with the Nanoscience Initiative at the Advanced Science Research Center at the CUNY Graduate Center (CUNY ASRC), the University of Pennsylvania, and the University of California-Merced took a unique approach that advances the opportunity to use mechanochemistry in large-scale production. The technique uses organic chemistry and nanotechnology to push molecules together and create chemicals without the use of costly solvents that pollute the environment. The research team’s findings have major implications for numerous manufacturing sectors, including the production of
pharmaceuticals and materials for a variety of medical and industrial purposes.
“This is a really exciting breakthrough, because the discovery makes mechanochemistry a reliable means of producing chemicals, and it allows us to do so without the harmful byproducts and large energy demands of current manufacturing techniques,” said the study’s lead author Adam Braunschweig, a professor of Chemistry and Biochemistry with the CUNY ASRC Nanoscience Initiative and Hunter College Department of Chemistry.
“When we pushed on the molecules, we found that they twisted into new, more reactive shapes that require less energy to combine and produce a desired chemical,” said first author Yerzhan Zholdassov, a doctoral student with the Braunschweig Lab. The experiment allowed researchers to measure the amount of force needed to create a predictable and reliable chemical reaction and show that mechanochemistry is a viable and scalable technique for manufacturing chemicals in a more sustainable, cost-efficient manner. The new technique can also be used to create new drugs and materials that can’t be created using current techniques that rely on solvents.
Co-author Robert Carpick, John Henry Towne Professor in the department of Mechanical Engineering and Applied Mechanics at the University of Pennsylvania’s School of Engineering and Applied Science who collaborated on this project, added: “This discovery was not possible without chemists teaming up with mechanical engineers in a truly cross-disciplinary way. The chemists were critical to designing and conducting the experiments, but we had to combine their forefront chemistry knowledge with advanced mechanics analysis to understand – through experiments and theory – how mechanical forces are accelerating chemical reactions here. The teamwork made the difference.”
Li Yuan, a current doctoral student and Alejandro Boscboinik, a former postdoctoral student in Carpick’s Research Group within the department of Mechanical Engineering and Applied Mechanics co-authored this research with him.
This research was funded by the National Science Foundation (NSF) Center for the Mechanical Control of Chemistry with additional support from the NSF Division for Innovation in Biological Research.
