Penn Researchers Show that Cells’ Perception of Stiffness is a Matter of Time
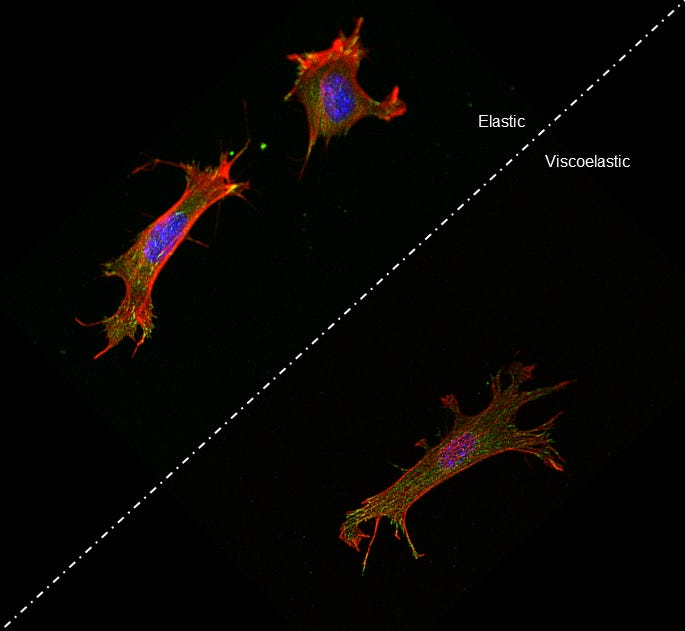
The relative stiffness of a cell’s environment is known to have a large effect on that cell’s behavior, including how well the cell can stick or move. Now, a new study by University of Pennsylvania researchers demonstrates the role timing plays in how cells perceive this stiffness.
Previous modeling studies have treated the extracellular matrix (ECM) as an elastic solid, but in reality, the ECM is partly viscous. Like mayonnaise or toothpaste, it has a mix of solid and liquid traits and is therefore viscoelastic. Most importantly, the stiffness of a viscous material changes when it is pushed or pulled on, and that change depends on the speed at which those deformations are applied. And just as different viscous materials have different intrinsic rates of relaxation, different cell types exert forces at different speeds.
The Penn team’s study shows that the closer those two timescales match, the more the cell is able to spread out on a surface. This kind of spreading is what allows cells to exert force in the first place, so it is a key quality for determining their role in a variety of diseases, such as cancer.
The study, published in the Proceedings of the National Academy of Sciences, was led by Vivek Shenoy, professor in the School of Engineering and Applied Science’s Department of Materials Science and Engineering and Director of the Center for Engineering Mechanobiology (CEMB), along with Ze Gong, a member of Shenoy’s lab.

The CEMB is a National Science Foundation Science and Technology Center, established to investigate this type of interplay between cells and the mechanical properties of their environments. Fellow CEMB members Robert Mauck, Mary Black Ralston Professor For Education And Research In Orthopaedic Surgery in Penn’s Perelman School of Medicine and Penn Engineering’s Department of Bioengineering, Paul Janmey, professor in Penn Medicine’s Department of Physiology and Penn Engineering’s Department of Bioengineering, and Jason Burdick, professor in Bioengineering, also contributed to this study. They collaborated with Ovijit Chaudhuri, an assistant professor of Mechanical Engineering at Stanford Univeristy.
“Most mechanobiology research has studied cells on materials that were solid like a sheet of rubber,” Shenoy said. “However, most extracellular matrices in tissue exhibit viscous behavior. That is, the tissue requires force to stretch, but over time, it holds the new shape with less resistance, similar to Silly Putty.”
This property of viscosity means time necessarily plays a role in how cells sense a viscous material’s stiffness. The rate at which viscoelastic materials relax has an intrinsic timescale, which is determined by the material’s initial stiffness, its final stiffness, and how long it takes to get between the two.
Cells also have an intrinsic timescale that governs how they apply force, which has to do with internal chemical processes, such as the rate at which certain proteins bind and unbind. Since this timescale differs between cells of different types, and between healthy and diseased cells, understanding the fundamentals of this interplay will be critical for future diagnostics and treatments.
Shenoy and his colleagues developed a computational model where both timescales can be independently controlled, allowing them to simulate whether cells sense this viscous behavior of the extracellular matrix.
“We found that the cell response is dependent on the rate at which the material relaxes. Only if the timing of the relaxation matches certain biological timescales do the cells maximize their response,” Shenoy said. “If the relaxation is fast, the cells don’t sense the viscosity at all, as if the material was only as stiff as its final stiffness. If the relaxation is slow, then the cells also perceive a substrate that has no viscosity, albeit at the higher initial stiffness.”
The researchers validated their computer model by observing cell movement on different surfaces whose elastic and viscous properties could be independently controlled. The more the timescales were in sync, the more these cells spread out and made contact with the surface.
“Making a lot of connections with the substrate means that cells are able to exert a lot of force, which is needed for movement,” Shenoy said. “This information provides a deeper understanding of how cells respond to real extracellular matrices, which has important implications for wound healing, fibrosis, and tumor growth.”
Future CEMB studies will involve developing even more detailed models, which capture deeper nuances in the extracellular matrix’s material properties.
The research was supported by the National Cancer Institute (CA193417) the National Institute of Biomedical Imaging and Bioengineering (EB017753)
Spencer Szczesny, and member of the Mauck lab, Steven Caliari, a member of the Burdick lab, and Elisabeth Charrier, a member of the Janmey lab, also contributed to the study
