When electronics need their own power sources, there are two basic options: batteries and harvesters. Batteries store energy internally, but are therefore heavy and have a limited supply. Harvesters, such as solar panels, collect energy from their environments. This gets around some of the downsides of batteries but introduces new ones, in that they can only operate in certain conditions and can’t turn that energy into useful power very quickly.
New research from the University of Pennsylvania’s School of Engineering and Applied Science is bridging the gap between these two fundamental technologies for the first time in the form of a “metal-air scavenger” that gets the best of both worlds.
This metal-air scavenger works like a battery, in that it provides power by repeatedly breaking and forming a series of chemical bonds. But it also works like a harvester, in that power is supplied by energy in its environment: specifically, the chemical bonds in metal and air surrounding the metal-air scavenger.
The result is a power source that has 10 times more power density than the best energy harvesters and 13 times more energy density than lithium-ion batteries.
In the long term, this type of energy source could be the basis for a new paradigm in robotics, where machines keep themselves powered by seeking out and “eating” metal, breaking down its chemical bonds for energy like humans do with food.
In the near term, this technology is already powering a pair of spin-off companies. The winners of Penn’s annual Y-Prize Competition are planning to use metal-air scavengers to power low-cost lights for off-grid homes in the developing world and long-lasting sensors for shipping containers that could alert to theft, damage or even human trafficking.

The researchers, James Pikul, assistant professor in the Department of Mechanical Engineering and Applied Mechanics, along with Min Wang and Unnati Joshi, members of his lab, published a study demonstrating their scavenger’s capabilities in the journal ACS Energy Letters.
The motivation for developing their metal-air scavenger, or MAS, stemmed from the fact that the technologies that make up robots’ brains and the technologies that power them are fundamentally mismatched when it comes to miniaturization.
As the size of individual transistors shrink, chips provide more computing power in smaller and lighter packages. But batteries don’t benefit the same way when getting smaller; the density of chemical bonds in a material are fixed, so smaller batteries necessarily mean fewer bonds to break.
“This inverted relationship between computing performance and energy storage makes it very difficult for small-scale devices and robots to operate for long periods of time,” Pikul says. “There are robots the size of insects, but they can only operate for a minute before their battery runs out of energy.”
Worse still, adding a bigger battery won’t allow a robot to last longer; the added mass takes more energy to move, negating the extra energy provided by the bigger battery. The only way to break this frustrating inverted relationship is to forage for chemical bonds, rather than to pack them along.
“Harvesters, like those that collect solar, thermal or vibrational energy, are getting better,” Pikul says. “They’re often used to power sensors and electronics that are off the grid and where you might not have anyone around to swap out batteries. The problem is that they have low power density, meaning they can’t take energy out of the environment as fast as a battery can deliver it.”
“Our MAS has a power density that’s ten times better than the best harvesters, to the point that we can compete against batteries,” he says, “It’s using battery chemistry, but doesn’t have the associated weight, because it’s taking those chemicals from the environment.”
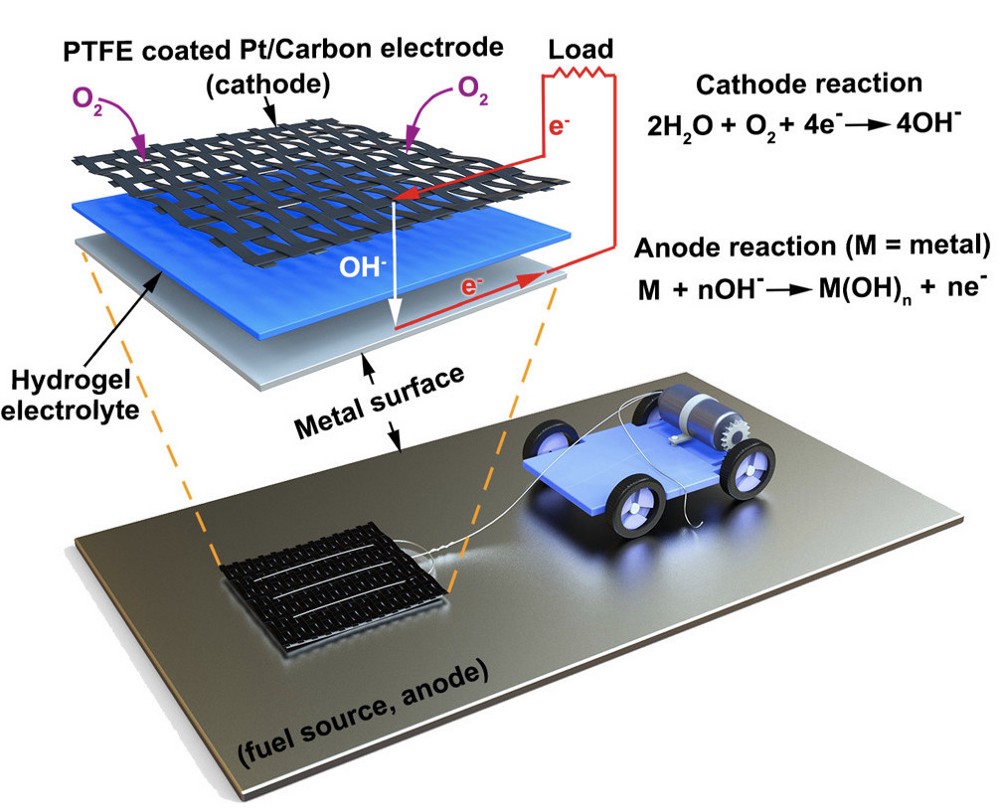
Like a traditional battery, the researchers’ MAS starts with a cathode that’s wired to the device it’s powering. Underneath the cathode is a slab of hydrogel, a spongy network of polymer chains that conducts electrons between the metal surface and the cathode via the water molecules it carries. With the hydrogel acting as an electrolyte, any metal surface it touches functions as the anode of a battery, allowing electrons to flow to the cathode and power the connected device.
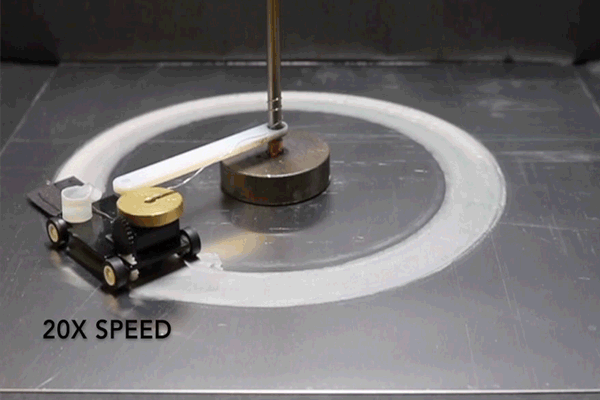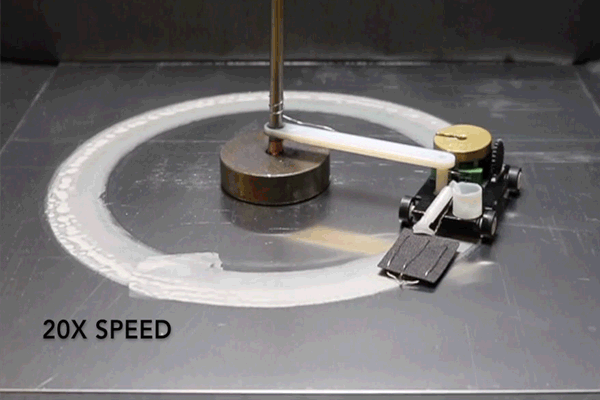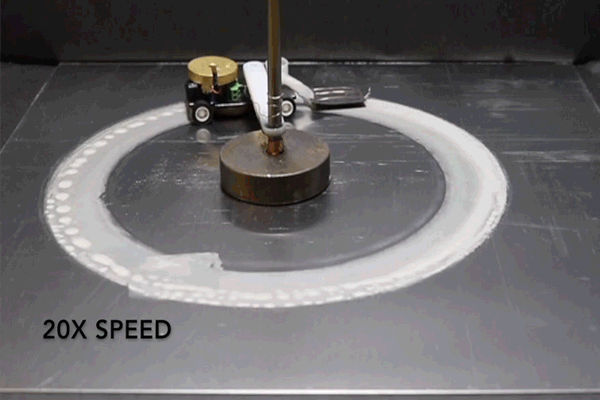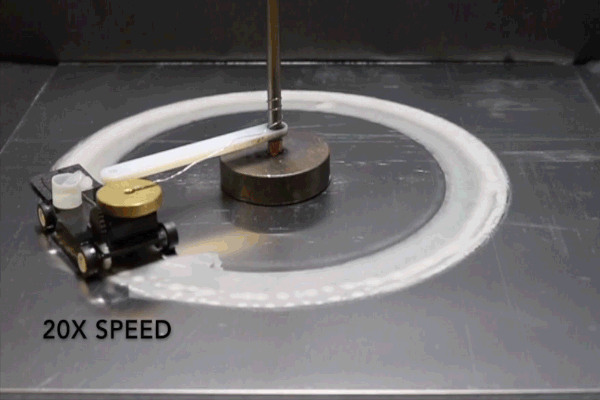
For the purposes of their study, the researchers connected a small motorized vehicle to the MAS. Dragging the hydrogel behind it, the MAS vehicle oxidized metallic surfaces it traveled over, leaving a microscopic layer of rust in its wake.
To demonstrate the efficiency of this approach, the researchers had their MAS vehicle drive in circles on an aluminum surface. The vehicle was outfitted with a small reservoir that continuously wicked water into the hydrogel to prevent it from drying out.
“Energy density is the ratio of available energy to the weight that has to be carried,” Pikul says. “Even factoring in the weight of the extra water, the MAS had 13 times the energy density of a lithium ion battery because the vehicle only has to carry the hydrogel and cathode, and not the metal or oxygen which provide the energy.”
The researchers also tested the MAS vehicles on zinc and stainless steel. Different metals give the MAS different energy densities, depending on their potential for oxidation.
This oxidation reaction takes place only within 100 microns of the surface, so while the MAS may use up all the readily available bonds with repeated trips, there’s little risk of it doing significant structural damage to the metal it’s scavenging.
With so many possible uses, the researchers’ MAS system was a natural fit for Penn’s annual Y-Prize, a business plan competition that challenges teams to build companies around nascent technologies developed at Penn Engineering. This year’s first-place team, Metal Light, earned $10,000 for their proposal to use MAS technology in low-cost lighting for off-grid homes in the developing world. M-Squared, which earned $4,000 in second place, intends to use MAS-powered sensors in shipping containers.
“In the near term, we see our MAS powering internet-of-things technologies, like what Metal Light and M-Squared propose,” Pikul says. “But what was really compelling to us, and the motivation behind this work, is how it changes the way we think about designing robots.”
Much of Pikul’s other research involves improving technology by taking cues from the natural world. For example, his lab’s high-strength, low-density “metallic wood” was inspired by the cellular structure of trees, and his work on a robotic lionfish involved giving it a liquid battery circulatory system that also pneumatically actuated its fins.
The researchers see their MAS as drawing on an even more fundamental biological concept: food.
“As we get robots that are more intelligent and more capable, we no longer have to restrict ourselves to plugging them into a wall. They can now find energy sources for themselves, just like humans do,” Pikul says. “One day, a robot that needs to recharge its batteries will just need to find some aluminum to ‘eat’ with a MAS, which would give it enough power to for it work until its next meal.”
This work was supported by the Office of Naval Research, grant N00014–19–1–2353. It was carried out in part at the Singh Center for Nanotechnology, which is supported by the NSF National Nanotechnology Coordinated Infrastructure Program under grant NNCI-1542153.
