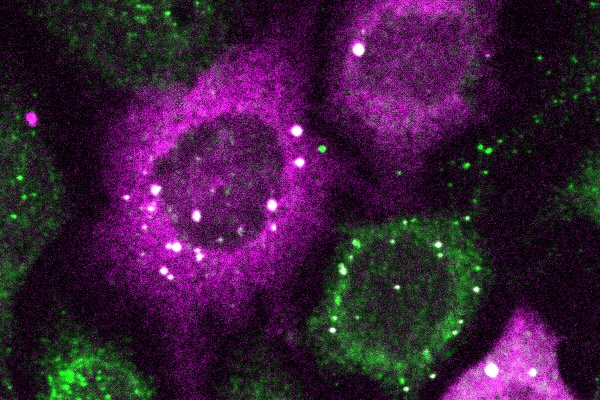
Penn Engineers have pioneered a new way to visualize the smallest protein clusters, skirting the physical limitations of light-powered microscopes and opening new avenues for detecting the proteins implicated in diseases like Alzheimer’s and testing new treatments.
In a paper in Cell Systems, Lukasz Bugaj, Assistant Professor in Bioengineering, describes the creation of CluMPS, or Clusters Magnified by Phase Separation, a molecular tool that activates by forming conspicuous blobs in the presence of target protein clusters as small as just a few nanometers. In essence, CluMPS functions like an on/off switch that responds to the presence of clusters of the protein it is programmed to detect.
Normally, says Bugaj, detecting such clusters requires laborious techniques. “With CluMPS, you don’t need anything beyond the standard lab microscope.” The tool fuses with the target protein to form condensates orders of magnitude larger than the protein clusters themselves that resemble the colorful blobs in a lava lamp. “We think the simplicity of the approach is one of its main benefits,” says Bugaj. “You don’t need specialized skills or equipment to quickly see whether there are small clusters in your cells.”
For treating diseases like Alzheimer’s, ALS and even cancer, being able to detect protein clusters this small promises to be a foundational advancement, allowing researchers to determine whether or not drugs actually eliminate disease-causing clusters of a target protein in a cell.
“You need a very clear signal,” says Bugaj, to know whether or not a treatment worked. “It’s very obvious when you have a gigantic cluster, but if you have small clusters, it is much harder. Now we can amplify that signal and see which drugs actually dissolve the clusters.”
In addition to providing new avenues for drug discovery, CluMPS will permit researchers to understand the functioning of proteins in new ways, leading to a deeper, more sophisticated rendering of cells themselves. “There’s an entire landscape of protein clustering that’s happening at the small scale, that’s important, but we just don’t know about it yet,” says Bugaj.
One of the challenges that CluMPS overcomes is that lightwaves themselves are larger than the smallest protein clusters, making it very hard to see such clusters without specialized techniques. “The wavelength of blue light is about 400 nanometers,” says Bugaj. “You can’t actually resolve the location of anything smaller than half that wavelength with a conventional microscope,” rendering protein clusters tens of nanometers wide all but invisible.
To develop CluMPS, Bugaj and his lab partnered with Elizabeth Rhoades, Professor of Chemistry at Penn Arts & Sciences, whose lab helped validate that CluMPS did indeed detect target protein clusters instead of generating false positives. “It was a really rewarding collaboration for us,” says Rhoades, “because it allowed us to apply the methods commonly used by our lab to help validate this powerful new tool in living cells. It was exciting to see how well we could differentiate between clusters and the single proteins.”
Thomas R. Mumford, a doctoral student in the Bugaj Lab and the paper’s lead author, played a key role in brainstorming and performing the necessary experiments. “It was crucial to characterize how underlying features of protein clusters interacted with CluMPS to trigger condensation,” says Mumford, to enable future users of the technology to understand precisely how it works. “The burden was on us to demonstrate that we were in fact detecting small clusters,” adds Bugaj. “One of the most rewarding aspects was working with Tom and the Rhoades lab to think of new types of experiments that would convincingly make the point.”
This study was conducted at the University of Pennsylvania School of Engineering and Applied science and supported by grants from The National Institutes of Health awarded to Bugaj (R35GM138211) and to collaborator Juan Guan (R35GM146877). Additional co-authors include Diarmid Rae, Abbas Idris, David Gonzalez-Martinez and Ayush Aditya Pal at Penn Engineering; Emily Brackhahn at Penn; and Michael C. Chung and Juan Guan at the University of Florida.
