Geometry Plays an Important Role in How Cells Behave, Penn Researchers Report
Inspired by how geometry influences physical systems such as soft matter, researchers at the University of Pennsylvania have revealed surprising insights into how the physics of molecules within a cell affect how the cell behaves.
“Cells have a skeleton just like we have a skeleton,” said Nathan Bade, a graduate student in the Department of Chemical and Biomolecular Engineering in the School of Engineering and Applied Science, “and, just like our skeleton, it’s stiff. We wanted to understand how that stiff skeleton would respond to geometry.”
The researchers focused on vascular smooth muscle cells, which are the types of cells that make up a large portion of large blood vessels in mammals. According to Bade, scientists might expect the cell to try to avoid bending. However, the researchers found that on a cylindrical surface the cells actually form very bent skeletons. They also found that, by manipulating the skeleton of the cells, they could recapitulate the alignment pattern of the skeleton that they saw in vivo.
“The most exciting thing we found is that geometry really matters when it comes to cell behaviors,” Bade said. “I think it’s something that has been somewhat overlooked compared to stiffness and other important environmental factors.”
The research was led by Bade, working under the guidance of Kathleen Stebe, the Richer & Elizabeth Goodwin Professor in the Department of Chemical and Biomolecular Engineering and deputy dean for research and innovation; Randall Kamien, the Vicki and William Abrams Professor in the Natural Sciences in the Department of Physics and Astronomy in the School of Arts and Sciences; and Richard K. Assoian, professor of pharmacology in Penn’s Perelman School of Medicine. Their paper was published in Science Advances.
“We already know that mammalian cells interact with boundaries,” Stebe said. “For example, if cells are grown on surfaces of different stiffness, they organize differently. That made us become interested in this question of geometry: Can a cell see the shape of its boundary? And we focused our initial work on cylindrical-like structures because they’re so common in biology.”
To investigate this, Bade coated cylinders with molecules that make them adhere to cells and then watched and gathered information about how the cells behaved when they grew on a curved boundary. The researchers used a powerful confocal microscope that provided them with three-dimensional information about the systems.
The researchers were able to treat the stress fibers, the active cytoskeleton within the cells, so that they would fluoresce. Using a laser to collect light from very small sections of a sample, the confocal microscope eliminated all the out-of-focus light. This produced a high-resolution image from a narrow plane which allowed the researchers to see that the population of stress fibers sitting on top of the cell was aligned differently from another population beneath.
They found that the size of the cylinder affected the cell’s response: The larger the cylinder, which results in a more planar geometry, the less the stress fibers align. Since smaller cylinders have larger curvature, the stress fibers aligned more strongly around them.
“One population of stress fibers aligns along the axis, and the other one wraps around the cylinder,” Stebe said. “There’s a very distinct pattern; it’s not subtle. So then we asked why this was happening.”
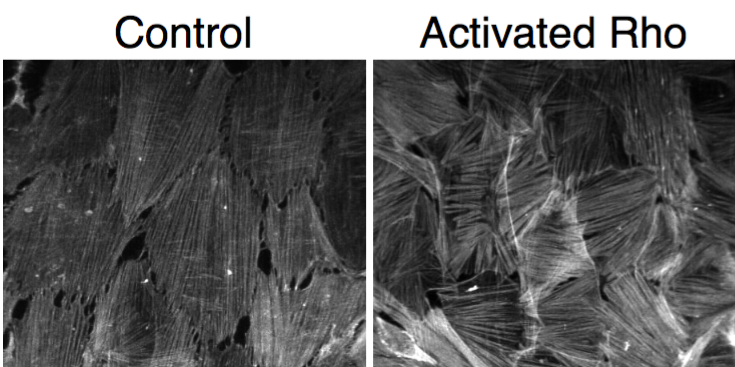
Using a drug specifically designed to activate Rho within cells and make the stress fibers thicker and potentially stiffer, the researchers set out to see if this increase in stiffness would discourage the stress fibers from wrapping around the cylinder. But, to their surprise, the researchers found that this treatment completely eliminated the fibers aligned along the axis and thickened the wrapped fibers.
“The reorganization is very striking,” said Stebe. “We think of it as the cells doing calculus; the cells sense and respond to the underlying curvature. Apparently, curvature is a cue that is playing a very strong role both in the organization of the cell itself and of the microstructure within the cell. These stress-fiber populations can be manipulated using drugs that change the stiffness, among other things. And, after these manipulations, the stress fibers maintain very strong alignments. This is not the usual argument for pattern formation in biology.”
Continue reading at Penn News.
