Drops of Liquid Crystal Molecules Branch Out Into Strange Structures
Shaped by surface tension and elasticity, spherical drops of chain-like liquid crystal molecules transform upon cooling into complex shapes with long-reaching tendrils.
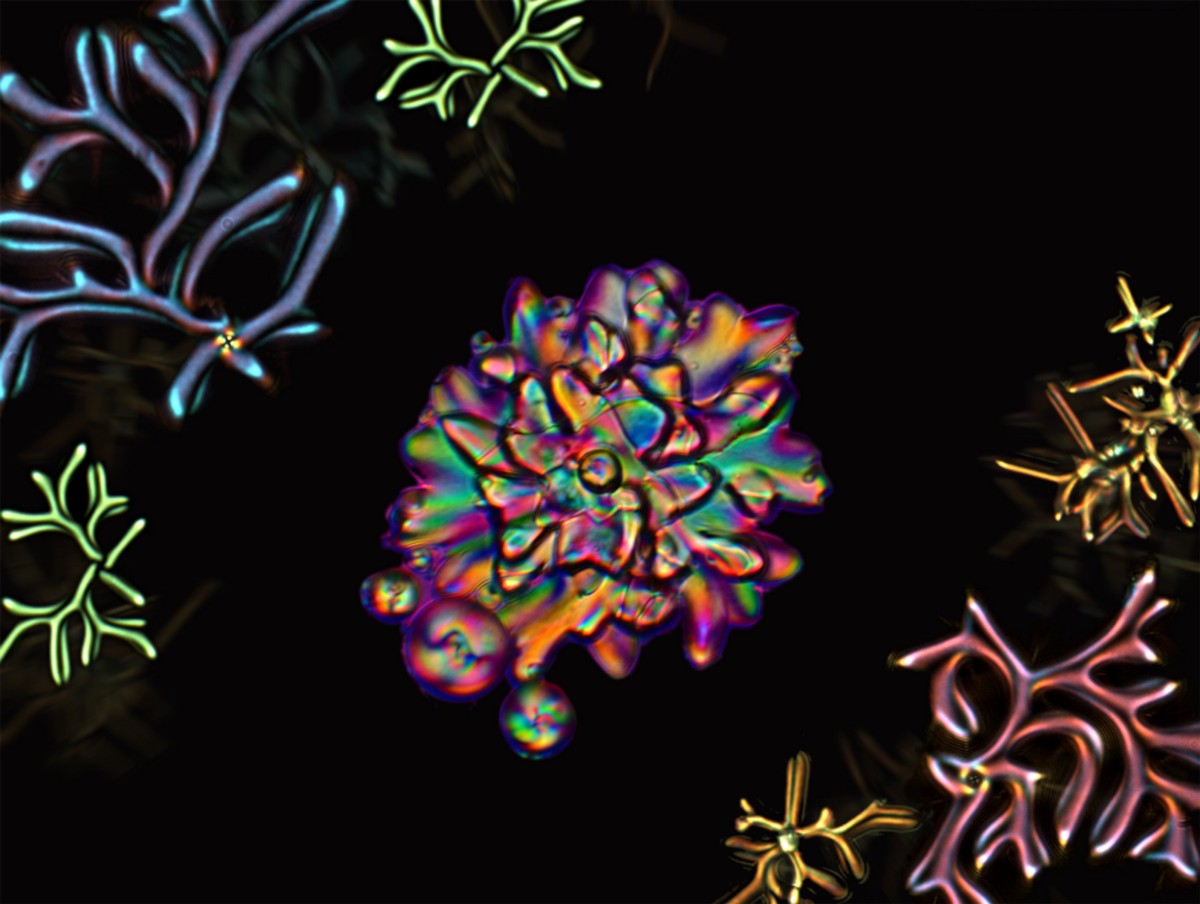
While many scientific achievements come from long years of careful planning, once in a while researchers stumble onto something completely unexpected. “At the beginning, we were looking to create a particular effect,” says graduate student Wei-Shao Wei. “Then, we observed something weird.”
A new study in Nature details this “weird” finding by showing how droplets containing chain-like liquid crystal molecules transform into complex shapes when the temperature drops. Conducted by Wei, graduate student Sophie Ettinger, Ph.D. alum Yu Xia, Shu Yang, and Arjun Yodh, this unexpected discovery provides new understanding about how molecular polydispersity — a condition where the lengths of liquid crystal molecules vary widely — can drive simple droplets to change into unusual shapes.

Unexpected results
Liquid crystals are composed of rod- or disc-like molecules called mesogens, and, as a result of the alignment of these mesogens, exhibit remarkable physical properties in between those of a solid and a liquid. The liquid crystals used in this study have similar characteristics to the ones used in LCD screens but are instead made of oligomers, flexible short-chain polymers comprised of smaller rod-like molecular building blocks.
Wei’s initial goal was to use this type of liquid crystal from Yang’s lab to create Janus droplets, which contain two different types of materials on opposite sides of the drop — in this case, one half would be a rubbery network called a liquid crystal elastomer, made by “locking” liquid crystal molecules in place with cross-linking, and the other half would be silicone.
Wei quickly discovered that the droplets were instead transforming into strange filamentous structures. At first the researchers thought that what they were seeing was an experimental error, but because the results were repeatable, they realized it was something remarkably new that they should try to understand.
“It was a visually spectacular effect. I wasn’t expecting it at all,” says Yodh. “We were trying to make designer drops, but in the process, we saw something interesting and different.”
Both amazed and puzzled by their strange results, the researchers began a rigorous investigation to explain what they were seeing. With the help of the Yang lab, Wei studied droplets that contained different mixtures of liquid crystal oligomers made of mesogens of varying lengths. The researchers varied oligomer chain length, used different surfactants to hold the droplets together, and explored simple theoretical models to make sense of their findings.
Continue reading at Penn Today.
