Nanocrystals are a type of inorganic nanoparticle, defined as any material with at least one dimension smaller than 100 nanometers. While nanocrystals have been used to create new types of devices, from digital displays to medical diagnostics, ongoing challenges related to their synthesis, characterization, and combining different types of nanocrystals into a single material need to be addressed to create new materials for more complex applications.
Now, a new paper in Science Advances, provides a blueprint for designing multifunctional materials using different combinations of nanocrystals. By bringing together theory, computational simulations, chemical synthesis, and assembly, researchers from the University of Pennsylvania and the University of Michigan demonstrate how an “inverse design” strategy can create unique materials from nanocrystals of varying compositions, sizes, and shapes. This work is an essential step towards establishing a “blueprint” for synthesizing new materials with unique properties.
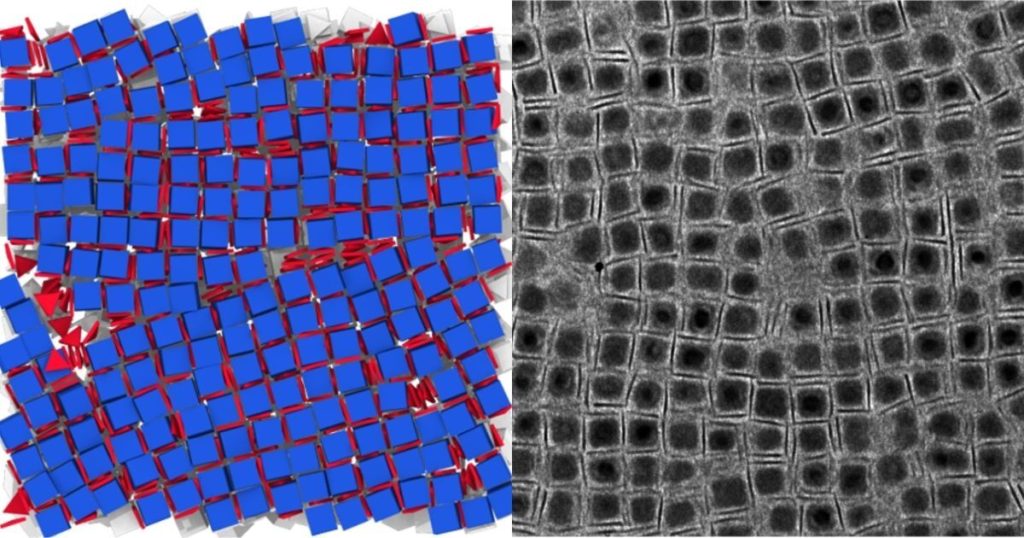
One of the challenges of using nanocrystals is finding ways to combine building blocks with different properties or chemical structures together to make something new. “It’s one of those problems where ‘like likes like,’” says recent Ph.D. graduate Katherine Elbert, who led this study while working in the lab of Penn Integrates Knowledge Professor Christopher Murray. “The different sizes and shapes of nanocrystals often segregate to form heterogeneous aggregates rather than ordered solids. Here, we’re trying to overcome that barrier and make materials in which the nanocrystals are precisely coupled to their neighbors to hybridize their properties.”
One of the greatest challenges in the area of research is the sheer number and types of nanocrystals. With massive libraries of nanocrystals that have varying chemical formulas, sizes, and shapes, it’s difficult for researchers to know exactly where to start. Searching such a large chemical space efficiently and systematically requires a multidisciplinary approach, which was made possible by a long-running collaboration between Murray and Sharon Glotzer’s computational modeling group at the University of Michigan. Their collaboration aims to establish design rules and to create blueprints that bring together multiple properties simultaneously.
“When dealing with complex, multicomponent architectures, putting each ‘brick’ exactly in the right place would be insurmountable,” says Murray. “But if you can find the rules by which nature wants to assemble nanocrystals and you know how to optimize the conditions and the precise design of blocks, you now have that blueprint for making different classes of materials.”
Continue reading at Penn Today.
