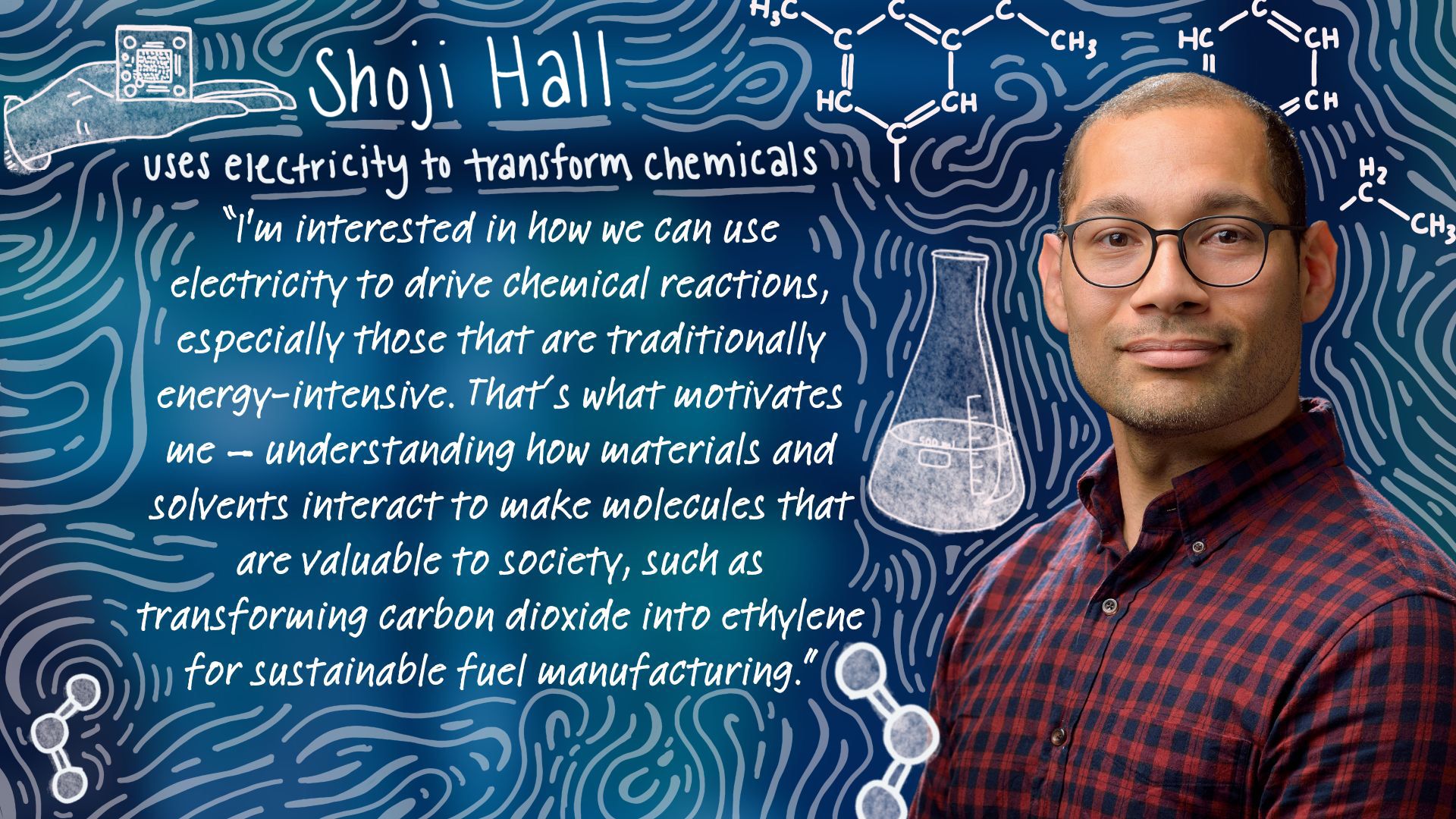
Shoji Hall, who joined Penn Engineering as an associate professor in Materials Science and Engineering (MSE) in January 2025, is focused on the chemistry of transformation: specifically, how electricity can be used to break and form chemical bonds at the interface between solids and liquids, a process called electrocatalysis. His work sits at the intersection of chemistry, materials science and sustainability.
“I’m interested in how we can use electricity to drive chemical reactions, especially those that are traditionally energy-intensive,” says Hall. “That’s what motivates me — understanding how materials and solvents interact to enable bond breaking and bond forming, and then using that knowledge to make molecules that are valuable to society, such as transforming carbon dioxide into ethylene for sustainable fuel manufacturing.”
By transforming carbon dioxide and nitrate into the molecular building blocks of fuel (carbon monoxide, methanol and ethylene) and fertilizer (ammonia), Hall is not only producing societally valuable molecules, he is finding a sustainable use for abundant “waste” molecules and one of the main greenhouse gas culprits of climate change.
A Path Shaped by Curiosity
Hall didn’t always know this was the path he’d take. As an undergraduate at UCLA, he started studying chemistry after his mother suggested a career in pharmacy.
“There wasn’t a pharmacy program at UCLA, so I did chemistry,” he laughs. “I wanted to be a little different from everyone doing biochemistry, and I ended up really enjoying it and quickly pursuing hands-on experience in a chemistry research lab as an undergrad.”
Hall’s research experience in an inorganic materials chemistry lab changed everything.
“This is where I first learned what materials science was and that it was its own field,” he says. “I loved it! I have stayed at the intersection of chemistry and materials ever since.”
Electrochemistry at the Interface
At the heart of Hall’s research is a deceptively complex space: the interface between a solid electrode and a liquid solvent. It’s here, at the molecular level, that electrochemical magic happens.
“Water is our most common solvent, but we still don’t have a deep understanding of how it affects the ability to make or break bonds,” Hall explains. “By studying this interface, we’re asking, ‘What is it about the material or the solvent that makes a reaction happen — or not?’
“It turns out the water itself plays a much bigger role than we thought,” he continues. “This kind of research brings us closer to developing cleaner, more efficient ways to make everyday materials from waste gases like CO or CO2, helping to reduce greenhouse emissions while producing things we still need.”
In addition to transforming carbon dioxide into more valuable molecules, Hall’s lab tackles some of the most pressing energy and environmental challenges by targeting reactions that could power the next generation of green technology. For example, one area of focus is fuel cell catalysis — specifically, improving the cathode side of hydrogen fuel cells, where oxygen is reduced to water.
“This reaction is where most of the energy is lost in the system,” Hall says. “If we can make that step more efficient, we can make fuel cells more viable as a sustainable energy option.”
Building a Bridge from Fundamentals to Application
At Penn Engineering, Hall’s lab is still taking shape, but once up and running it will be a space filled with potentiostats (tools used to control voltage and current), gas chromatography equipment (to analyze reaction products) and the telltale signs of dynamic, experimental work: glass flasks, compressed gas tanks and shelves lined with solvents and salts.
“Right now, we’re still in the design phase, building out the lab’s capabilities,” says Hall. “But the goal is to build upon and then take the fundamental systems we study and bridge them to more applied spaces like full-scale electrolyzers or industrial reactors to create plastics and fuel, as well as convert water into hydrogen as a source of green energy.”
Looking Ahead
For Hall, Penn Engineering provides the right mix of opportunity and infrastructure.
“The Singh Center has amazing characterization and fabrication tools, and there are a lot of people here, especially from Vagelos Laboratory for Energy Science and Technology, that I can collaborate with,” he says. “It’s an exciting place to build something new.”
Hall taught his first course on materials chemistry this past spring, and has been building out his lab, recruiting students and meeting colleagues in his first semester. He is recruiting a second Ph.D. student this fall and is also interested in working with master’s and undergraduate students.
What will success look like after this first year?
“A built-out lab and a group of strong, motivated students,” Hall says. “If I can help them learn how to be scientists and think like engineers, that’s a foundation for many years of impactful work ahead.”
Learn more about Hall’s work on his research website here.
