Penn Engineering secured a multi-million-dollar contract with Wellcome Leap under the organization’s $60 million RNA Readiness + Response (R3) program, which is jointly funded with the Coalition for Epidemic Preparedness Innovations (CEPI). Penn Engineers aim to create “on-demand” manufacturing technology that can produce a range of RNA-based vaccines.
The Penn Engineering team features Daeyeon Lee, Evan C Thompson Term Chair for Excellence in Teaching and Professor in Chemical and Biomolecular Engineering, Michael Mitchell, Skirkanich Assistant Professor of Innovation in Bioengineering, David Issadore, Associate Professor in Bioengineering and Electrical and Systems Engineering, and Sagar Yadavali, a former postdoctoral researcher in the Issadore and Lee labs and now the CEO of InfiniFluidics, a spinoff company based on their research. Drew Weissman of the Perelman School of Medicine, whose foundational research directly continued to the development of mRNA-based COVID-19 vaccines, is also a part of this interdisciplinary team.
The success of these COVID-19 vaccines has inspired a fresh perspective and wave of research funding for RNA therapeutics across a wide range of difficult diseases and health issues. These therapeutics now need to be equitably and efficiently distributed, something currently limited by the inefficient mRNA vaccine manufacturing processes which would rapidly translate technologies from the lab to the clinic.
The Penn team’s project aims to create a platform for on-demand distributed manufacturing of mRNA therapies that can address many diseases using a two-pronged approach. One thrust of the project, led by Mitchell, will use a design method that creates a barcode library of novel lipid nanoparticles (LNPs) with custom features, while the other, led by Yadavali, will develop microfluidic chips for the precise manufacturing of these RNA-based therapies.
The effectiveness of RNA-based therapeutics hinges on selecting both the right genetic sequences and the right LNPs to carry them into a patient’s cells. With a near-limitless range of customizations that can impact an LNP’s ability to encapsulate an mRNA strand or direct it to a particular type of cell, Mitchell’s lab has developed machine learning tools to optimize LNP design. Through deep sequencing and bioinformatics, Mitchell and Weissman will identify candidate LNPs which exhibit the best mRNA delivery then create an LNP library based on the sequences of the RNA cargo they carry, which will allow for differentiation amongst the many LNP+RNA pairs produced. They will then deploy these tagged LNP-packaged RNA sequences in mice models to quantify the molecules in mouse organs, tissues and cells.
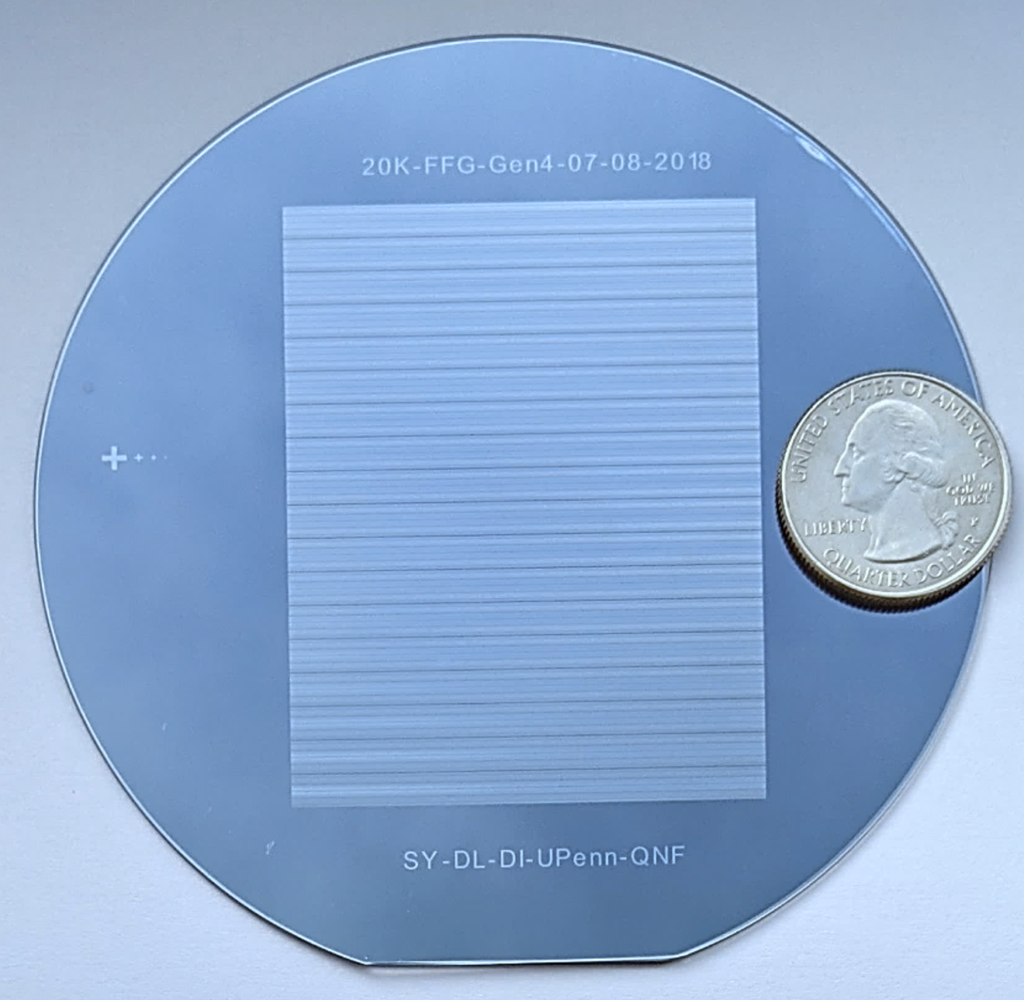
Once tagged with a barcode based on the sequences of the RNA, LNPs will be produced on a single four-inch silicon and glass chip known as a Very Large Scale Microfluidic Integration (VLSMI) platform, a technology developed by Yadavali, Lee and Issadore which addresses the hurdle of production time in RNA therapeutics. The system produces an effective flow rate that is more than ten thousand times faster than what can be typically achieved in a microfluidic device and will allow for high product quality, thanks to hundreds of LNP-producing units per chip.
Wellcome Leap builds and executes bold, unconventional programs that aim to deliver breakthroughs in human health over 5-10 years. The team at Penn’s previous research has already set them up for success to achieve the program’s goals, and the University’s facilitation of collaborations, frequent in-person interactions, and exchange of ideas and materials, as well as its wealth of innovators and researchers, will continue to provide the team with the tools it needs to accomplish accelerated work in RNA therapeutics.
