The rise of drug-resistant bacteria infections is one of the world’s most severe global health issues, estimated to cause 10 million deaths annually by the year 2050. Some of the most virulent and antibiotic-resistant bacterial pathogens are the leading cause of life-threatening, hospital-acquired infections, particularly dangerous for immunocompromised and critically ill patients. Traditional and continual synthesis of antibiotics will simply not be able to keep up with bacteria evolution.
To avoid the continual process of synthesizing new antibiotics to target bacteria as they evolve, Penn Engineers have looked at a new, natural resource for antibiotic molecules.

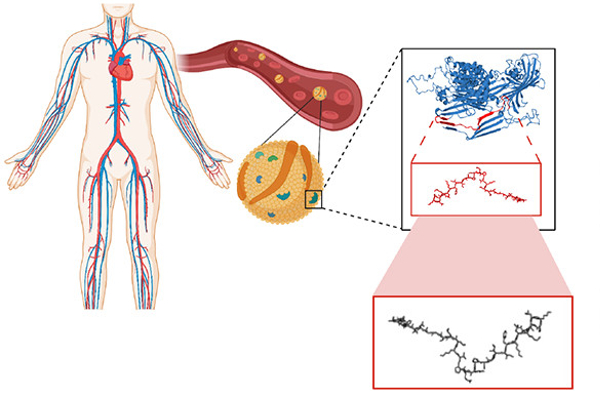
A recent study on the search for encrypted peptides with antimicrobial properties in the human proteome has located naturally occurring antibiotics within our own bodies. By using an algorithm to pinpoint specific sequences in our protein code, a team of Penn researchers along with collaborators, led by César de la Fuente, Presidential Assistant Professor in Psychiatry, Bioengineering, Microbiology, and Chemical and Biomolecular Engineering, and Marcelo Torres, a post doc in de la Fuente’s lab, were able to locate novel peptides, or amino acid chains, that when cleaved, indicated their potential to fend off harmful bacteria.
Now, in a new study published in ACS Nano, the team along with Angela Cesaro, the lead author and post doc in de la Fuente’s lab, have identified three distinct antimicrobial peptides derived from a protein in human plasma and demonstrate their abilities in mouse models. Angela Cesaro performed a great part of the activities during her PhD under the supervision of corresponding author, Professor Angela Arciello, from the University of Naples Federico II. The collaborative study also includes Utrecht University in the Netherlands.
“We identified the cardiovascular system as a hot spot for potential antimicrobials using an algorithmic approach,” says de la Fuente. “Then we looked closer at a specific protein in the plasma.”
Apolipoprotein B is a protein in blood plasma that carries lipids, such as cholesterol, throughout the body. However, when this protein is broken down, its peptide building blocks exhibit different functions altogether.
Using their algorithm, the team isolated three peptides from Apolipoprotein B and tested their ability to fend off different types of bacteria, including those that cause staph infections and pneumonia.
Each of the three peptides were able to permeate the cytoplasmic membrane of the bacteria, kill the cell, and impede the growth of biofilms. Additionally, when used in conjunction with each other or with pharmaceutical antibiotics, their antibiotic effect increased significantly, requiring a smaller dosage to fight infection.
The team also assessed whether these peptides promote antibiotic resistance in these bacteria.
“There are many ways our immune cells and antimicrobial peptides attack and fight off bacterial infection,” says de la Fuente. “What is unique in the peptides we are examining is their ability to attack the bacterial membrane, a structure that requires multiple genes to build and maintain. Typical antibiotics only target one gene or aspect of bacterial cells making it relatively easy for bacteria to develop resistance, so antimicrobials such as the peptides we describe here that attack multiple targets at once are more successful at impeding bacterial resistance.”
“In the resistance evolution experiment we performed in our lab, it was surprising to see how quickly new bacteria resistant to common antibiotics are selected, and, on the contrary, how the encrypted peptides discovered within the plasma do not lead to this type of selection,” says Cesaro. “This behavior might derive from a host-defense mechanism developed in humans and evolutionarily conserved over time. This work opens new avenues for antimicrobial discovery in proteins unrelated to the immune system and this is very exciting since at the current time new antibiotics are badly needed.”
In fact, it is the physiochemical properties of the bacterial membrane itself that allows for the peptides to be so successful in this fight.
“Peptides act rapidly on the membranes of invading bacteria through different mechanisms,” says Torres. “In this case, the bacterial membranes act as magnets, attracting the antimicrobial peptides, and because these membrane properties are complex and cannot be easily altered to avoid peptide attraction, the bacteria are consequently overcome by antimicrobial peptides and destroyed, with multiple hurdles in the way of developing any resistance in the next generation.”
Removing the potential of resistance means that these peptides could be used as antibiotis for a wide range of bacterial infections and remain efficient longer than traditional antibiotics.
Finally, to increase stability for testing the antimicrobial function in vivo, one peptide was designed, synthesized, and used in a mouse model. The experiment showed a skin bacterial infection treated with the synthetic peptide based on the natural antibiotics identified from Apolipoprotein B, which was able to eradicate the infection in four days with a single dose.
“The blood was a clear place to look for encrypted peptides as determined by the algorithm, and these results provide a link between human plasma proteins and our innate immunity,” says de la Fuente. “We continue to look for these peptides beyond the blood, in all other sites of the body to provide that link with the nervous, digestive and immune systems as well.”
