When asked to think of a chemical reaction, you might picture bubbling liquids in a beaker, or maybe applying heat to a mixture until something transforms. But some of the most important reactions in nature and industry don’t need heat or solvents. Instead, they need force.
Mechanochemistry is where physical pressure or stress triggers chemical reactions. Imagine molecules being rammed together like bumper cars, or shaken up in a giant cocktail shaker. That shaking and colliding happens every day inside car engines, manufacturing equipment and experimental green reactors. But until recently, scientists have struggled to explain exactly how these force-fueled reactions happen or how to make them work better. At Penn Engineering, the laboratory of Robert Carpick, John Henry Towne Professor in Mechanical Engineering and Applied Mechanics (MEAM), was looking at this issue as members of the Center for the Mechanical Control of Chemistry, a National Science Foundation-funded Chemical Center of Innovation that aims to transform the understanding and adoption of mechanochemistry.
Now, Carpick, along with Cangyu Qu, postdoctoral researcher, and Lu Fang, a former Ph.D. student in the Carpick lab, has developed a theoretical model that overcomes previous challenges in accurately describing the relationship between mechanical stress and chemical reactions. Their recent study, published in APS’ Physical Review B, fills in the gap for describing the forces that occur when molecules are squeezed between two surfaces. This result helps make it easier to predict mechanochemical reactions, which are promising for the green manufacturing of plastics, metallic compounds, lubricants and more.
Previous Limitations in Mechanochemistry
Scientists have long known that mechanical stress can “activate” chemical reactions, meaning it can lower the energy needed to get molecules to react. But previous efforts to measure a key property called the “activation volume,” which tells us how readily the applied stress changes the energy needed for a reaction to happen, have produced wildly inconsistent results.
“Different studies were showing activation volumes that differed by 100-fold,” explains Qu. “This makes it challenging for researchers studying mechanochemistry to interpret these kinds of reactions. We couldn’t reliably determine either the magnitude or the physical meaning of activation volume across different scenarios.”
Without an accurate way to measure activation volume, engineers couldn’t confidently predict mechanochemical reactions, impeding the design of better lubricants, cleaner industrial processes or more efficient material synthesis techniques. Mechanochemistry holds promise for greener chemistry — skipping the need for high heat or polluting solvents — but it cannot be a viable option for large-scale synthesis of chemicals or manufacturing of materials without better tools to predict the outcomes.
Two Spheres, One Breakthrough
To untangle the confusion, the team took a close look at how to measure these reactions in the lab and at the simplest scale possible. They examined what was happening at the point of contact between two spheres: Think ball bearings pushed together, but much smaller.
“But the stresses at contacting points aren’t perfectly uniform,” says Carpick. “When two spheres touch, the stress isn’t the same across the whole contacting area. That throws off the math and this conundrum became our first challenge to overcome.”
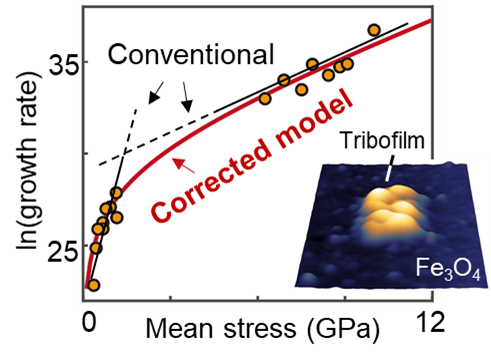
Previous models made assumptions about the stresses and the size of the contact, which led to inaccuracies that could be so large, they would be useless in any optimization study. Carpick’s team developed a new model that corrects for two key issues: First, the stress across the contact isn’t uniform, and second, as you press the spheres harder together, the area of contact changes, which affects how many molecules are actually reacting.
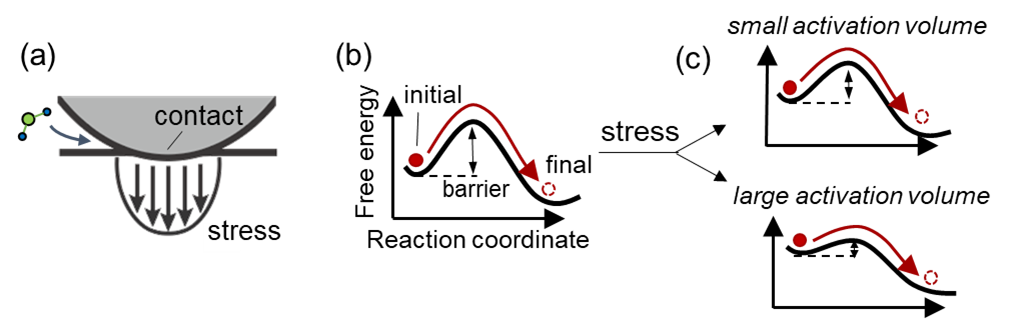
By incorporating both of these factors, the researchers produced a model that lines up neatly with real-world data, resolving longstanding discrepancies between micro-scale and nano-scale experiments.
A Simpler System, a Greener Future
To validate their model, the team studied reactions in a system called a Hertzian contact, a classic way to describe how round objects touch. Rather than modeling complex industrial equipment directly, they focused on a simpler setup: a spherical tip pressing against a flat surface. That’s a good stand-in for one collision at a time inside something like a ball mill, a real-world mechanical reactor that works kind of like a giant cocktail shaker for molecules. This type of sphere-on-flat contact also represents the basic building block of almost all practical rough surfaces, where contact occurs at many tiny, individual points that closely resemble this geometry.
“What we found,” says Qu, “is that by accounting for both stress distribution and contact area, we could finally unify the data that used to look totally scattered. All those strange activation volume values now agreed, and made sense.”

From Engines to Energy Efficiency
The implications of this model go well beyond the lab bench.
In one direction, it helps engineers design better lubricants for engines, especially in gas-powered and electric vehicles. Inside an engine, special additives in the lubricants that protect the moving parts react under stress to form protective films that reduce wear. But until now, designing those additives has involved a significant amount of trial and error.
“Mechanochemistry can help us balance performance and efficiency,” says Fang. “If a lubricant is too thick, it wastes energy. If it’s too thin, it doesn’t protect the engine or gears. With better data, we can pick the right additives that react just enough under pressure to do both.”
In another direction, the model unlocks new possibilities in green manufacturing. Mechanochemical synthesis can create everything from inorganic and organic compounds to polymers, nanoparticles, energy-related materials and pharmaceuticals, often without the need for heat or organic solvents — two major sources of energy consumption and waste in traditional chemistry. But industry adoption has been slow because of uncertainty around how to control the reactions. This new model helps change that.
“With a more complete understanding, we can tune the activation volume,” Carpick says. “We want to be able to choose or even design molecules for given mechanical conditions that give us the reactions we want in a clean, efficient and precise way. This model helps us get closer to that grand challenge.”
Future Mechanochemical Tools
While the model focuses on simplified systems, the team sees it as a building block for more complex, practical applications. Future versions could simulate actual reactors, model different material surfaces or guide the development of entirely new classes of chemical products or materials.
“We finally are gaining a clear window into the primary way that mechanical stress drives chemistry,” Carpick says. “And that will mean we can stop just shaking the cocktail and hoping for the products we want, and instead we can engineer it with previously unachievable precision.”
This work was supported by the Center for the Mechanical Control of Chemistry under Grant No. CHE-2303044, and the NSF Grant No. CMMI-184702. This work was carried out in part at the Singh Center for Nanotechnology, which is supported by the NSF National Nanotechnology Coordinated Infrastructure Program under Grant No. NNCI- 2025608.
