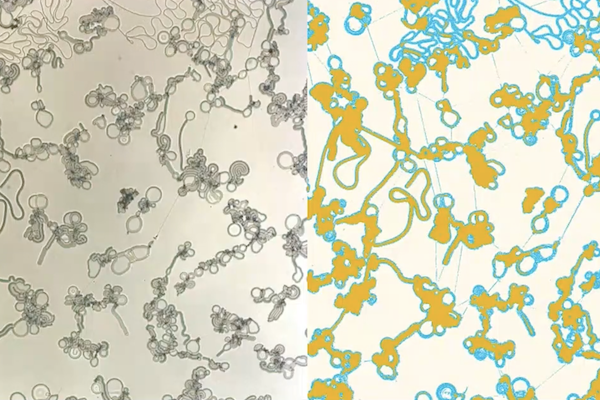
Liquid crystals are all around us, from cell phone screens and video game consoles to car dashboards and medical devices. Run an electric current through liquid crystal displays (LCDs) and they generate colors, thanks to the unique properties of these fluids: rearrange their shape, and they reflect different wavelengths of light.
As the lab of Chinedum Osuji, Eduardo D. Glandt Presidential Professor and Chair of Chemical and Biomolecular Engineering, recently discovered, these fascinating molecules may be able to do even more. Under the right conditions, liquid crystals condense into astonishing structures, spontaneously generating filaments and flattened discs that can transport material from one place to another, much like complex biological systems.
The insight may lead to self-assembling materials, new ways to model cellular activity and more. “It’s like a network of conveyor belts,” says Christopher Browne, a postdoctoral researcher in Osuji’s lab and the co-first author of a recent paper in Proceedings of the National Academy of Sciences (PNAS) that describes the finding. “It was this serendipitous observation of something that superficially looks very lifelike — that was the initial cue that this might be something more general and more interesting.”
Browne and Osuji are now part of an NSF-supported interdisciplinary group at the Laboratory for Research on the Structure of Matter (LRSM) led by Matthew Good, Associate Professor of Cell and Developmental Biology within the Perelman School of Medicine, and Elizabeth Rhoades, Professor of Chemistry within the School of Arts & Sciences, that is studying condensate formation in biological and non-biological systems.
Originally, Osuji’s lab partnered with ExxonMobil to investigate mesophase pitch, a substance used in the development of high-strength carbon fibers, like those found in Formula 1 cars and high-end tennis rackets. “Those materials are liquid crystals,” says Osuji, of the chemical precursors to the carbon fibers themselves. “Or better stated, they are liquid crystalline over some period of their existence during processing.” While experimenting with condensates at different temperatures, Yuma Morimitsu, another postdoctoral fellow in the Osuji Lab and the paper’s other co-first author, noticed unusual behavior in the material.
Normally, if you combine two immiscible — that is, not mixable — fluids and then heat them to a high enough temperature to force them to mix, if you then cool the mixture, at some point it will separate or “demix.” Typically, this happens by the formation of droplets that coalesce to form a separate layer, much like how, if you combine oil and water, you eventually wind up with a layer of oil on top of the water.
In this case, the liquid crystal, 4’-cyano 4-dodecyloxybiphenyl, also known as 12OCB, spontaneously formed highly irregular structures when separating from squalane, a colorless oil. “Instead of forming drops,” says Osuji, “when you have this phase separation between the liquid crystal and the other components of the system, you form cascaded structures, the first of which is these filaments, which grow rapidly and thereafter form another set of structures — what we call bulged discs or flat droplets.”
To understand the system, the researchers used powerful microscopes to observe the liquid crystals’ movement on the micrometer scale — that is, millionths of a meter, comparable to the width of a human hair. “The first time we saw these structures, we looked at them at a cooling rate that was excessively high,” recalls Osuji, leading the liquid crystals to clump together. Only by lowering the cooling rate and further zooming in did the researchers realize that the liquid crystals were spontaneously forming structures reminiscent of biological systems.
Interestingly, Browne found, a number of researchers had come close to observing similar behavior decades ago, but either studied systems in which the behavior was not particularly pronounced, or lacked microscopy powerful enough to visualize what was actually happening.
For Browne, the result’s most exciting implication is that it brings together several traditionally disparate fields: the world of active matter research, which focuses on biological systems that transport material and produce motion, and the realms of self-assembly and phase behavior, which study materials that create new structures on their own and that behave differently when changing phase. “This is a new type of active matter system,” says Browne.
He and Osuji also point to the possibility of leveraging the finding to emulate biological systems, either to better understand how they work or to manufacture materials. “Molecules are being absorbed into the filaments and then shuttled into those flat droplets continuously,” says Osuji, “even though just by looking at the system, you can’t discern any obvious activity.” In effect, the flat droplets could function like small reactors, churning out molecules that the filaments carry to other droplets for storage or further chemical activity.
The researchers also suggest that their finding could reinvigorate research into liquid crystals themselves. “When a field becomes industrialized,” says Browne, “oftentimes the fundamental research tapers off. But sometimes there are lingering puzzles that nobody finished solving.”
This study was conducted at the University of Pennsylvania, in the School of Engineering and Applied Science’s Department of Chemical and Biomolecular Engineering and the School of Arts & Sciences’ Department of Physics and Astronomy, and ExxonMobil’s Research Division. The work was supported by a grant from ExxonMobil and by the U.S. National Science Foundation (DMR-2309043).
Additional co-authors include Zhe Liu of Penn Engineering; Paul G. Severino of the School of Arts & Sciences; and Manesh Gopinadhan, Eric B. Sirota, Ozcan Altintas and Kazem V. Edmond of ExxonMobil.
