Most organisms have proteins that react to light. Even creatures that don’t have eyes or other visual organs use these proteins to regulate many cellular processes, such as transcription, translation, cell growth and cell survival.
The field of optogenetics relies on such proteins to better understand and manipulate these processes. Using lasers and genetically engineered versions of these naturally occurring proteins, known as probes, researchers can precisely activate and deactivate a variety of cellular pathways, just like flipping a switch.
Now, Penn Engineering researchers have described a new type of optogenetic protein that can be controlled not only by light, but also by temperature, allowing for a higher degree of control in the manipulation of cellular pathways. The research will open new horizons for both basic science and translational research.

Lukasz Bugaj, Assistant Professor in Bioengineering (BE), Bomyi Lim, Assistant Professor in Chemical and Biomolecular Engineering, Brian Chow, Associate Professor in BE, and graduate students William Benman in Bugaj’s lab, Hao Deng in Lim’s lab, and Erin Berlew and Ivan Kuznetsov in Chow’s lab, published their study in Nature Chemical Biology. Arndt Siekmann, Associate Professor of Cell and Developmental Biology at the Perelman School of Medicine, and Caitlyn Parker, a research technician in his lab, also contributed to this research.
The team’s original aim was to develop a single-component probe that would be able to manipulate specific cellular pathways more efficiently. The model for their probe was a protein called BcLOV4, and through further investigation of this protein’s function, they made a fortuitous discovery: that the protein is controlled by both light and temperature.
“Light-activated proteins are a new kind of research tool that have increased the precision in how we study and understand cell function,” says Bugaj. “Our original aim was to create simpler, more effective such tools to control two separate signaling pathways that are central to cell physiology and are commonly implicated in cancer.”
These cellular signaling pathways, Ras and PI3K, play a role in regulating cell death, so a better understanding of them may help us learn how healthy cells react to disease and why tumor cells continuously grow and spread.
Traditional probes for these pathways typically involve two distinct optogenetic proteins that can require tedious optimization of their relative amounts in a cell. While such optimization is straightforward in individual cells, it is much more difficult when looking at tissues or entire organisms.
“Compared to previous probes, ours were based on a single protein called BcLOV4, which was recently described by Brian Chow’s lab,” says Bugaj. “As a single protein, BcLOV4 can stimulate signals in a manner that required multiple proteins in previous approaches, thus making it simpler and easier to use.”
The authors successfully showed that BcLOV4-based probes could stimulate the Ras and PI3K pathways in mammalian cells, as well as in zebrafish and fruit flies, two common model organisms.
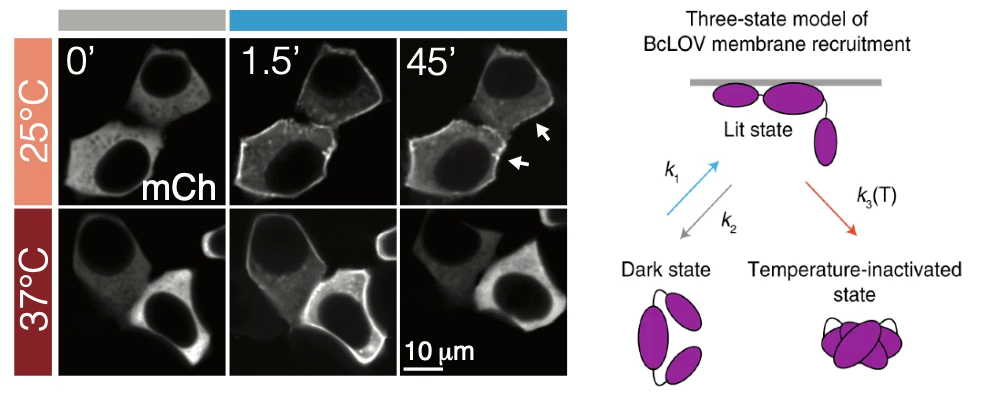
“However, in the course of our experiments, we serendipitously discovered that BcLOV4 could sense not only light, but also temperature,” says Bugaj. “As far as we know, this type of dual light and temperature sensitivity is a completely new feature for photosensory proteins.”
“The majority of our paper characterizes this dual light and temperature sensitivity, explores different experimental systems beyond mammalian cells where our tools can be applied, such as fly cells and developing zebrafish, and then describes new experimental capabilities that leverage light and temperature sensitivity,” he says.
When the team discovered the temperature control in this optogenetic protein, they modeled various light and temperature conditions to predict how the protein would respond. They also found that, by using temperature to deactivate BcLOV4, they could control multiple light-sensitive proteins independently within the same cell.
“For those who study photosensory proteins, our work presents an example of a protein whose activity responds to two distinct stimuli, light and temperature, and implies that other such proteins may exist,” says Bugaj. “This discovery also opens new possibilities for more sophisticated, multi-input remote control of cell function. Our work also has implications for the new field of thermogenetics, or cellular control using temperature, which is already being used as a remote control of engineered cell therapies in animal models.”
“The temperature-sensitive behavior of BcLOV4 is unlike any that has been described before,” he says. “The protein changes subcellular localization based on temperature and thus holds promise for a completely new class of temperature-responsive proteins with unique capabilities.”
