 The common procedures patients experience in a dentist’s office – from dental plaque removal to a root canal of an infected tooth – could look and feel very different in just a few years thanks to a team of Penn Engineers and clinicians at the Center for Innovation & Precision Dentistry (CiPD).
The common procedures patients experience in a dentist’s office – from dental plaque removal to a root canal of an infected tooth – could look and feel very different in just a few years thanks to a team of Penn Engineers and clinicians at the Center for Innovation & Precision Dentistry (CiPD).
Daeyeon Lee, Russell Pearce and Elizabeth Crimian Heuer Professor in Chemical and Biomolecular Engineering (CBE), Edward Steager, Research Assistant Professor in Mechanical Engineering and Applied Mechanics (MEAM), and Hyun (Michel) Koo, Professor in Dental Medicine and in Bioengineering, are transforming the field of dental care with their cutting-edge research into microrobots capable of navigating the complex environments of the mouth to deliver targeted treatments. From cleaning teeth to treating infections in hard-to-reach spaces like root canals, these tiny robots could pave the way for more effective, non-invasive oral care.
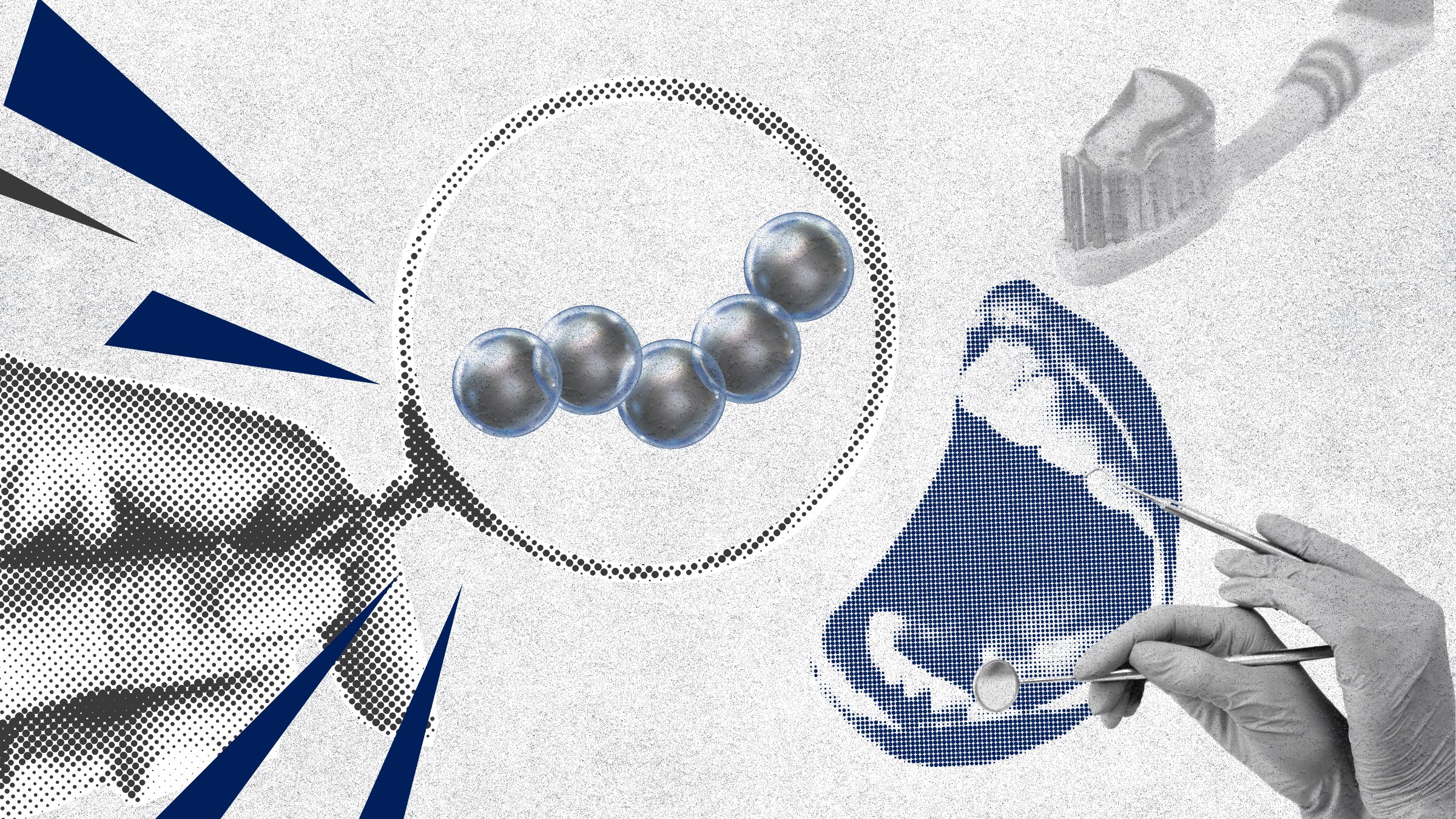
This interdisciplinary team’s work led to the invention of shapeshifting microrobots that can easily brush and floss teeth. In recent studies, led by Hong Huy Tran, a postdoctoral scholar in CiPD, these microrobots have gained greater locomotion to traverse a wide range of surfaces and difficult-to-access places in the mouth and an improved ability to deliver targeted therapies to specific locations.
Moving in New Ways to Pinpoint Treatment
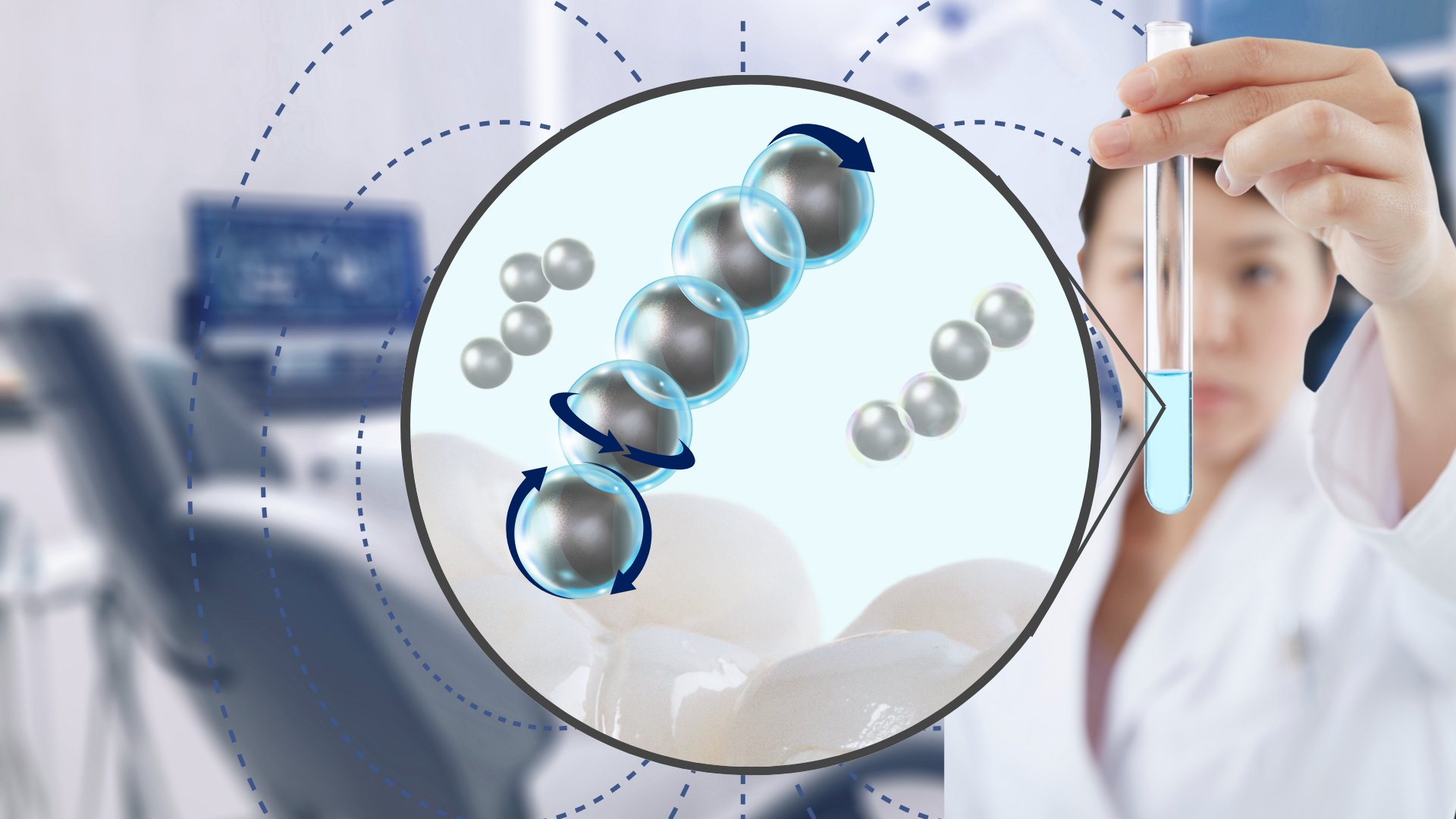
The team’s 2024 paper, published in Advanced Healthcare Materials, describes their new platform that integrates nanozyme-shelled microrobots, made using microfluidics templates that combine individual iron oxide and silica nanoparticles into 100-µm microcapsules roughly the width of a human hair. Inducing a magnetic field causes the microcapsules to self-organize into larger collectives, which can then reach the apical regions of the root canal. Once in place, the robots catalyze hydrogen peroxide to generate reactive oxygen species (ROS), effectively killing harmful biofilms on contact.
“This breakthrough is really about precision,” says Tran. “Apical periodontitis, a common inflammation due to an infection in the root canal, is hard to treat because root canals are incredibly narrow and hard to access. But with our microrobots, we can target these deep, difficult-to-reach areas with much greater accuracy. It’s a huge step forward in treating infections without invasive surgery.”
The 2025 follow-up paper, published in ACS Nano, takes what the team learned about microcapsules the previous year and makes them even better suited for getting to other tricky parts of the mouth. They introduce a new hierarchy of self-reconfiguring microrobots that can move across both hard and soft tissues and navigate through liquid and sticky substances like mucosal tissue, where biofilm formation is particularly challenging. This level of adaptability was achieved by carefully engineering the microcapsules to create stable, yet adaptable collective structures that “walk” through the mouth, avoiding obstacles and traversing complex terrains.
“We’ve created a new way to assemble the robots,” explains Lee. “By combining iron oxide and silica nanoparticles in a specific arrangement, we’ve found a way to increase their collective movement while ensuring they remain stable in the mouth’s varying conditions. This hierarchical assembly allows the microrobots to adapt as they move through different environments, and we can control where they go with a standard set of electromagnets.”
Microrobots Beyond the Mouth
The ultimate goal is to enable clinicians to use these robots to deliver highly targeted treatments to areas of the mouth that are typically difficult to reach with traditional methods.
“Both studies are opening doors for engineers and roboticists to develop new approaches, and for the clinicians that will eventually use them,” says Koo. “Biofilms, whether in the mouth or elsewhere in the body, are difficult to treat with a single approach due to their varied compositions, environments and the tissue types they colonize.
“Now, these microrobots can get almost anywhere we need them to go and have the potential to target a variety of biofilms that lead to health issues, such as cavities and gum diseases, as well as fungal infections,” continues Koo. “I envision microrobots becoming a ‘high-precision multi-tool’ for dentists to perform a wide range of clinical procedures, from routine cleanings to complex root canal treatments.”
Applications extend beyond just the mouth. The team sees these therapy-carrying microrobots making their way into areas like orthopedic joints, the stomach and implants.
“The mouth is an accessible place in the body to deploy microrobots or check in on their progress in fighting an infection,” says Steager. “It’s also dynamic and ever-changing with hard, soft and even liquid environments. This makes it an excellent starting point for testing microrobots. The challenges we face here mirror many other complex environments in the body like skeletal joints and the stomach.”
In more immediate future steps, the team’s work has already caught the attention of the clinical community, and they are currently preparing for animal studies, including trials for biofilm treatment.
Emergent Solutions, Collaboration and the Next Generation of Problem Solvers
With clinical trials on the horizon, the team’s collaborations with clinicians have been key to refining the microrobots’ design and functionality.
“This work is a great example of what can happen when engineers and clinicians collaborate closely,” says Lee. “It’s about making sure that the technology we create is not just innovative, but also practical and applicable in real-world medical settings. The feedback from clinicians has been invaluable in guiding our research.”
“At CiPD, we are transforming oral health innovations with engineering solutions – advancing cutting-edge research and training the next-generation interdisciplinary leaders,” adds Koo. “Together with Kate Stebe (CiPD’s co-director), and our exceptional faculty and trainees, we’ve opened the door to bold, unconventional thinking. These partnerships are sparking breakthroughs that I believe will revolutionize oral and craniofacial health care. Before this, we couldn’t have imagined microrobots performing precision tasks in the most inaccessible areas in the oral cavity.”
These studies add to the CiPD’s larger collection of work and are incorporated into the curriculum as the team mentors incoming students.
“We are constantly taking what we learn together through research into the classroom,” says Steager. “These kinds of studies help students visualize real-world applications of engineering and seemingly distant solutions, which can be made accessible with a fundamental understanding of chemical, biomolecular and mechanical engineering that they are gaining as they pursue their degrees.”
By combining cutting-edge engineering with clinical insight, Penn’s team is not only pioneering a new era of oral health care, where precision, adaptability and targeted treatments can lead to better patient outcomes, they are also ensuring emerging leaders in the field will be equipped with the tools needed to conduct future interdisciplinary collaboration required to drive these solutions forward.
Read more about the team’s research on the CiPD website.
The research in this story was funded by the National Institute for Dental and Craniofacial Research grants: R01 DE025848, R01 DE031491 and R56 DE029985.
