
From smartphones to wind turbines, rare earth elements (REEs) are an essential part of the hardware in many advanced technologies. These elements, which include the lanthanides along with scandium and yttrium, are the backbone of industries that rely on unique properties such as luminescence, magnetism and catalytic ability. In fact, as our world moves toward more sustainable energy solutions and increasingly sophisticated technologies, the demand for REEs is projected to grow substantially.
There’s one catch, however: REEs are difficult to extract and even harder to separate. These elements, despite their name, aren’t actually rare in terms of abundance. What makes them “rare” is their dispersion throughout the Earth’s crust and their chemical similarities, which make them incredibly challenging to isolate from one another. Current separation methods — largely reliant on toxic solvents like kerosene — are not only inefficient, but also harmful to people and the environment. Additionally, while the U.S. once dominated REE mining and production, environmental restrictions on current separation methods have limited domestic production.
Kathleen Stebe, Richer & Elizabeth Goodwin Professor in Chemical and Biomolecular Engineering (CBE), is tackling this challenge head-on with a collaborative group of researchers across five institutions under the support of a grant from the Department of Energy. Stebe is leading a groundbreaking research initiative that aims to create an eco-friendly, bioinspired process for separating REEs that would also avoid shipping semi-processed REEs to other countries for purification.
“Current separation methods use kerosene and extractants-molecules that bind the REE cations, a positively charged particle, that create issues, both environmentally and in terms of efficiency,” says Stebe. “The separation process is not selective enough to efficiently separate lanthanides, meaning that it has to be repeated many times to achieve REEs in sufficient purity. The whole method is cumbersome and creates unnecessary waste.”
Stebe, along with a team of researchers from Penn, the City College of New York, the University of Illinois Chicago, Northwestern University and the University of Chicago, look to human biology to find the molecule best suited for the job of separation: peptides.
Bioinspired Interfaces: Drawing on Nature’s Expertise
In nature, organisms have evolved proteins that selectively bind to specific ions, despite their similar properties. A perfect example of this is calcium-binding proteins in the human body, which can distinguish between calcium and magnesium ions, even though both have the same charge.
“We are applying this concept to create a similar level of selectivity for rare earth elements,” says collaborator E. James Petersson, Professor of Chemistry, Biochemistry and Biophysics at Penn’s School of Arts & Sciences. “By using peptide-based molecules — specifically, a truncated version of the EF-hand motif, which is naturally found in calcium-binding proteins — we are designing molecules that can selectively bind to specific rare earth elements.”
This EF-hand motif refers to the structure and mechanism through which these naturally occurring proteins and peptides are able to differentiate between two very similar molecules.
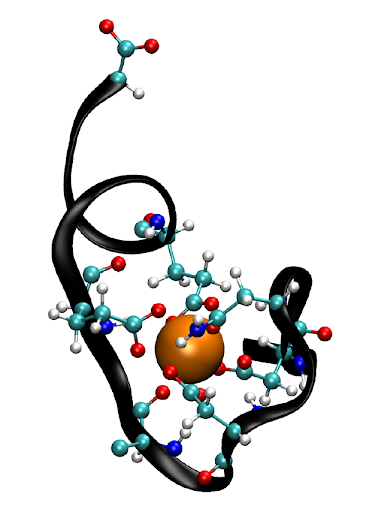
“The structure resembles a hand,” says Stebe, “and each ‘finger’ of the hand is laden with a carboxyl or carbonyl group that binds to cations floating around in solution. It’s a beautiful and complex structure that can recognize the nuanced and subtle differences between two nearly indistinct cations, and then capture and hold onto whichever cation it is ‘looking’ for. This is extremely important for separating REEs, which differ in size by only one-tenth of an Angstrom.”
In the team’s recent study published in PNAS, they found that EF-hand-containing peptides could bind to the peptide-cation complex and capture it at the aqueous-air interface. The vision includes using bubbles to separate specific lanthanides from a mixture. Once bound to the peptides in an aqueous solution, the REEs will rise to the surface, where they are trapped in a foam at the water-air interface, a separation technique called ion foam flotation.
“My primary area of research is in interfacial science, studying the adsorption of surface-active molecules — surfactants and soap molecules — to the air-water interface,” says co-author Charles Maldarelli, Professor of Chemical Engineering at The City College of New York. “This study gave me the opportunity to apply my expertise to the adsorption of peptides and peptide-metal complexes at the interface.”
Felipe Jimenez-Angeles, Research Associate Professor at Northwestern University, performed many of the molecular dynamics simulations in this study. “I am fascinated that these peptides can separate ions that only differ by a few tenths of an Angstrom in diameter via the differences in the electrostatic forces at the atomic scale. The water-soluble peptide reconfigures when it captures the ion and becomes insoluble in water, resulting in its adsorption to the air-water interface.”
The team’s next steps in this research will be investigating how to scale this process, allowing them to isolate target REEs and collect them at usable quantities in a way that is much more efficient and environmentally friendly.
The Collaborative Effort Behind the Innovation
What makes this project truly innovative is the collaboration across multiple universities and disciplines. Each institution brings unique expertise to the project, from synthetic chemistry to surface material properties, and even X-ray experiments.
“This is really the first time my lab has used biology to solve chemistry problems,” says Petersson. “Normally, we focus on creating chemical probes to study biology, often looking at neurodegenerative disorders like Parkinson’s disease. But the experience of working on this project has inspired me to explore other biological approaches to chemistry, including adapting disease-related proteins for applications in other fields like energy and sustainability.”
“I have long been interested in molecular interface interactions,” adds Ivan Dmochowski, Professor of Chemistry in Penn’s School of Arts & Sciences. “As an undergraduate, I made molecules that react with the surface of glass and gold, and studied the resulting monolayers that formed. Later I started looking at proteins at the air-water interface.”
Other key senior faculty involved in the research include Monica Olvera de la Cruz from Northwestern University, Raymond Tu from CCNY, Mark Schloassman from University of Illinois at Chicago, and Daeyeon Lee, Ravi Radhakrishnan and Cesar de la Fuente at the University of Pennsylvania.
“It has been rewarding to both contribute to and learn from this effort,” continues Domchowski. “To solve really challenging, societally relevant problems in 2025, we will need a wide range of technical expertise, and I am excited to continue working with this team of collaborators to do that.”
Looking Ahead: The Future of Rare Earth Element Recovery
As Stebe’s team continues their work, they are focused on fine-tuning the selectivity of the peptides and optimizing the process for bulk production. Their next steps include using specialized peptides designed by Petersson to enhance the fluorescence of the system, allowing for more precise tracking of the binding events. They also plan to use physics data to inform additional opportunities for improved specificity and look into developing new, synthetic molecules to make this method even more cost-effective and environmentally friendly.
“This is just the beginning,” says Stebe. “We have a lot of exciting new directions to explore, from using synthetic molecules instead of peptides to creating even more selective binding structures. The potential impact of this work goes far beyond just rare earth elements — it could revolutionize the way we approach material separation across many industries.”
