
Penn Engineers have discovered a novel means of directing lipid nanoparticles (LNPs), the revolutionary molecules that delivered the COVID-19 vaccines, to target specific tissues, presaging a new era in personalized medicine and gene therapy.
While past research — including at Penn Engineering — has screened “libraries” of LNPs to find specific variants that target organs like the lungs, this approach is akin to trial and error. “We’ve never understood how the structure of one key component of the LNP, the ionizable lipid, determines the ultimate destination of LNPs to organs beyond the liver,” says Michael J. Mitchell, Associate Professor in Bioengineering.
In a new paper published in Nature Nanotechnology, Mitchell’s group describes how subtle adjustments to the chemical structure of the ionizable lipid, a key component of the LNP, allows for tissue-specific delivery, in particular to the liver, lungs and spleen.
The Power of Siloxane
The researchers’ key insight was to incorporate siloxane composites, a class of silicon- and oxygen-based compounds already used in medical devices, cosmetics and drug delivery, into the ionizable lipids that give LNPs their name.
Much like silicon housewares, which are known for being durable and easy to sanitize, siloxane composites have been shown in prior research to have high stability and low toxicity. “We sought to explore if these attributes could be exploited to engineer highly stable and minimally toxic LNPs for mRNA delivery,” the researchers report in the paper.
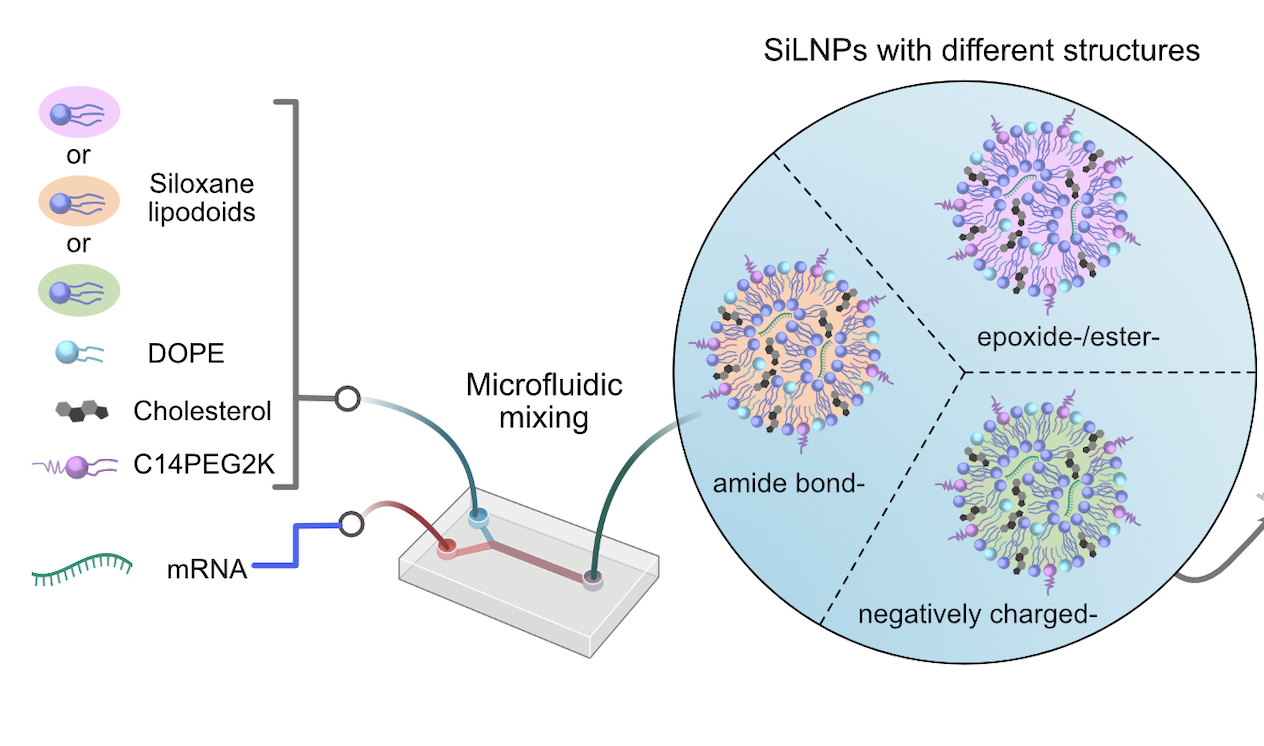
By carefully testing hundreds of variants of the newly christened siloxane-incorporating lipid nanoparticles (SiLNPs), the researchers determined which chemical features had an effect on mRNA delivery. “Identifying their in vivo delivery was a huge challenge,” says Lulu Xue, a postdoctoral fellow in the Mitchell Lab and one of the paper’s co-first authors.
Finding the Right Formula
At first, the researchers used the SiLNP variants to deliver mRNA encoding for firefly luciferase, the gene that causes fireflies to glow, to cancerous liver cells in an animal model, as a proxy for using SiLNPs to treat liver cancer. Wherever cells started to glow, the researchers could be confident that SiLNPs had transferred their mRNA payload to cells.
When glowing cells also appeared in the animal models’ lungs, the researchers realized that certain SiLNPs variants were guiding the molecules outside the liver — the holy grail of LNP research, since LNPs tend to congregate in the liver, due to that organ’s convoluted network of blood vessels.
Among the changes the group identified that adjusted the trajectory of the SiLNPs were adjustments as small as substituting one chemical group for another — an amide for an ester, in this case — which led to a 90% success rate in delivering mRNA to lung tissue in the animal model.
“We just changed the structure of the lipids,” says Xue, “but this small change in the lipid chemistry substantially increased extrahepatic delivery.”
New Effects, New Applications
The group also determined that a wide variety of chemical factors affected the SiLNPs’ overall efficacy, including the number of silicon groups in the lipids, the length of the lipids’ tails and the structure of the lipids themselves.
In addition, the SiLNPs had a marked affinity for endothelial cells; since blood vessels are made of endothelial cells, SiLNPs may have clinical applications in regenerative medicine that targets damaged blood vessels, in particular in the lungs. Indeed, the researchers found that SiLNPs delivering substances that promote new blood vessel growth dramatically improved blood oxygen levels and lung function in animal models suffering from a viral infection that damaged their lungs’ blood vessels.
The researchers theorized that one reason for SiLNPs’ effectiveness could be that silicon atoms are larger than carbon atoms. Because the atoms are less tightly packed, when SiLNPs fuse with target cell membranes, the former likely increases the fluidity of the latter. That extra flexibility in turn helps the mRNA carried by SiLNPs enter the target cell, so the mRNA can be used to produce proteins more readily. As the SiLNPs travel through the bloodstream, proteins that attach to their surface also help guide them to the right tissue.
The Future of Precision Medicine
Ultimately, the SiLNPs showed up to a sixfold improvement in delivering mRNA compared to the current gold-standard LNP varieties, suggesting that the unique properties of the siloxane composites have a pronounced effect on the molecules’ clinical potential. “These SiLNPs show promise for protein replacement therapies, regenerative medicine and CRISPR-Cas-based gene editing,” says Xue.
“We hope that this paper can lead to new clinical applications for lipid nanoparticles by showing how simple alterations to their chemical structure can enable highly specific mRNA delivery to the organ of interest,” adds Mitchell.
This study was conducted at the University of Pennsylvania’s School of Engineering and Applied Science (Penn Engineering), School of Veterinary Medicine (PennVet), Perelman School of Medicine (Penn Medicine); the University of Electronic Science and Technology of China; the University of Delaware; and Temple University and was supported by a U.S. National Institutes of Health (NIH) Director’s New Innovator Award (DP2 TR002776), a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), a U.S. National Science Foundation CAREER Award (CBET-2145491), an American Cancer Society Research Scholar Grant (RSG-22-122-01-ET), and the National Institutes of Health (NICHD R01 HD115877).
Additional co-authors include Ningqiang Gong (co-first author), Xuexiang Han, Sarah J. Shepherd, Rohan Palanki, Junchao Xu, Kelsey L. Swingle, Rakan El-Mayta, Il-Chul Yoon and Jingchen Xu of Penn Engineering; Gan Zhao (co-first author), Zebin Xiao and Andrew E. Vaughan, of PennVet; Vivek Chowdhary, Mohamad-Gabriel Alameh, Claude Warzecha, Lili Wang, James M. Wilson and Drew Weissman of Penn Medicine; Xinhong Xong and Jiaxi Cui of the University of Electronic Science and Technology of China; Darrin J. Pochan of the University of Delaware; and Karin Wang of Temple University.
