Penn Bioengineers: Cells Control Their Own Fate by Manipulating their Environment
By Lauren Salig
As different as muscle, blood, brain and skin cells are from one another, they all share the same DNA. Stem cells’ transformation into these specialized cells — a process called cell fate determination — is controlled through various signals from their surroundings.
A recent Penn Engineering study suggests that cells may have more control over their fate than previously thought.

Jason Burdick, Robert D. Bent Professor of Bioengineering, and Claudia Loebel, a postdoctoral researcher in his lab, led the study. Robert Mauck, Mary Black Ralston Professor for Education and Research in Orthopaedic Surgery at Penn’s Perelman School of Medicine, also contributed to the research.
Their study was published in Nature Materials.
The last few decades of biological research have uncovered the importance of studying the microenvironment that cells inhabit, including how the chemistry and mechanics of that environment impact cell behavior.
“We often use a class of engineered materials called hydrogels to mimic cellular environments and to probe their influence on cell behavior,” says Burdick.
Through hydrogels and other methods, research on the interplay between cells and their environment has made progress, but it still has a long way to go.
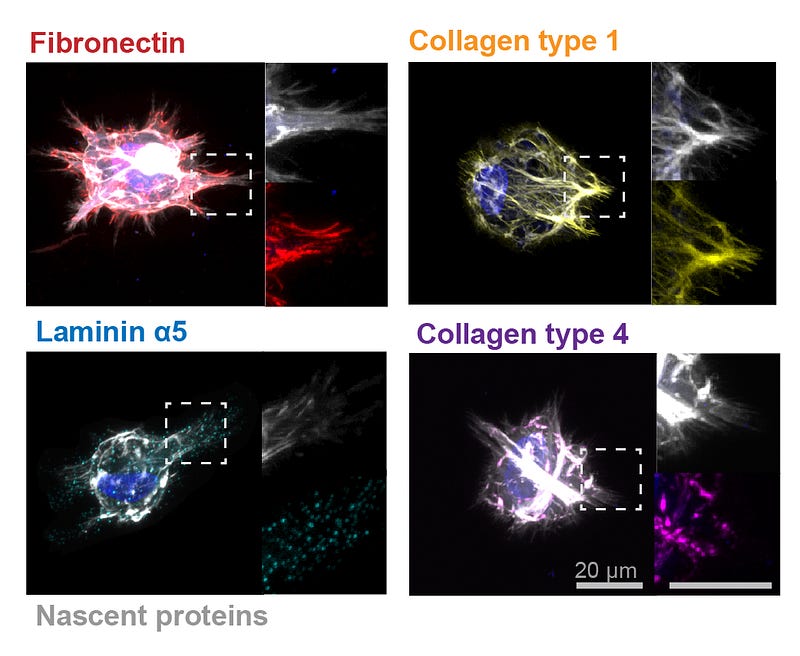
To begin addressing gaps in understanding, the Penn researchers studied the importance of the proteins that cells secrete into their environment on regulating aspects of their own behavior, including fate.
To investigate this cell-environment interaction, Burdick, Loebel and Mauck first had to develop some creative methods. The team designed a new imaging technique to visualize proteins that cells produced in their microenvironment. The researchers also developed two unique hydrogels with varied biophysical properties into which the cells were embedded and used in conjunction with their labeling technique.
“These hydrogels were engineered to represent many of the biophysical properties found in tissues in the body,” says Loebel.
The study found that cells began to secrete proteins within hours of being encapsulated in the hydrogels and that those proteins played an important role in changing the extracellular environment and regulating cells’ behavior, including cell fate determination. The cells essentially determined their own function by shaping their environment through proteins.
To determine the importance of these proteins on cell behavior, Burdick’s team blocked the cells’ ability to interact with the proteins they produced and their ability to break down those proteins. Blocking the communication between cells and their secreted proteins altered the outcome for the cells, including the way the cells spread, signaled and determined their fate.
The study’s finding that secreted proteins meaningfully impact cell behavior calls for a reevaluation of how hydrogels are used in the field. The biophysical properties of hydrogels are often implicated in cell behavior. Burdick, Loebel and Mauck’s work suggests that the proteins secreted by cells within hours after embedding within a hydrogel may supplement or even cancel out the effects of the inherent hydrogel properties that scientists are intending to study. The protein labelling technique developed in Burdick’s lab could help scientists better understand how and when proteins impact cell behavior.
Research like Burdick’s, which looks at the interaction between cells and their environment, provides insights that are important for the design of new materials in tissue engineering and drug screening. Understanding the cell and cellular environment, not just as individual entities but as an interwoven system, is crucial to progress in biological fields.
This work was supported by the Swiss National Foundation through an SNF Early Postdoc Mobility Fellowship, the National Science Foundation through DMR award 1610525, the Center for Engineering MechanoBiology through grant CMMI: 15–48571 and the National Institutes of Health through grant R01 EB008722.
